Question
Question: In which of the following hydrolysis reactions, esters and hydrolysis products are correctly matched...
In which of the following hydrolysis reactions, esters and hydrolysis products are correctly matched?
A.CH3CH2COOC2H5H2OH2SO4CH3CH2COOH+C2H5OH
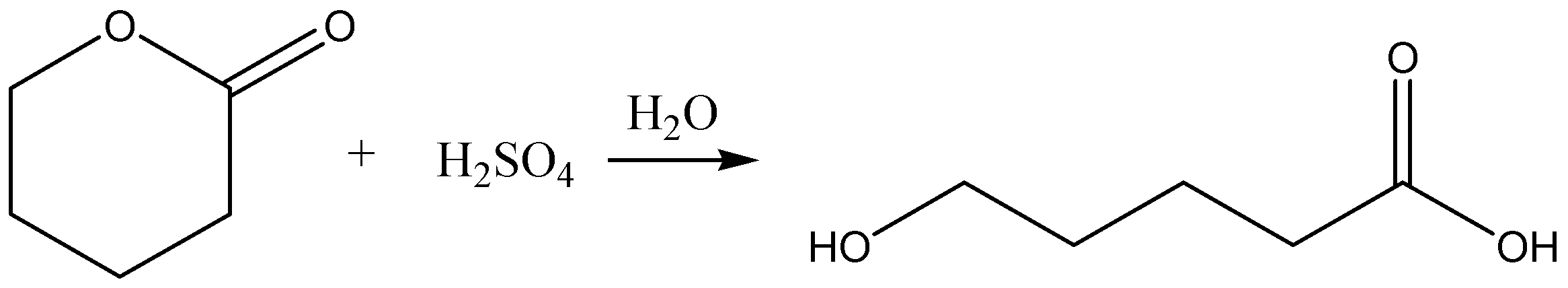
B.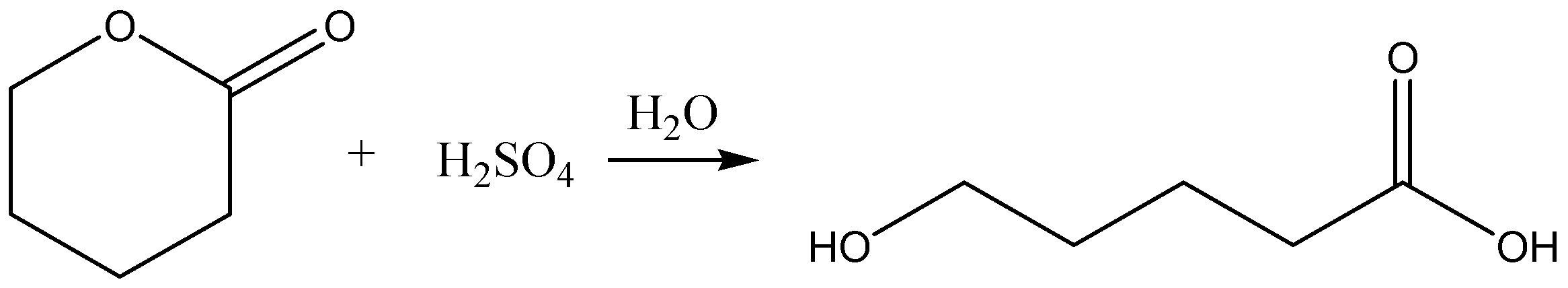
C.CH3CO−OCH2O−O=CCH3H2SO4H20CH3COOH+HCHO
D.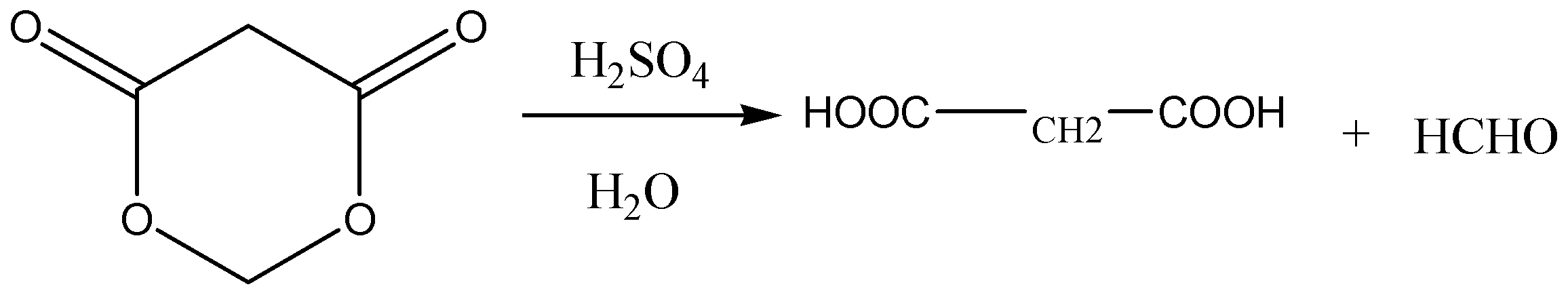
Solution
From the word hydrolysis we will understand that the question is talking about the water means the addition of water or reaction with water.
We know that hydrolysis reactions use water to interrupt down polymers into monomers and this is often opposite to dehydration synthesis, which sorts water when synthesizing a polymer from monomers. Hydrolysis reactions break bonds and release energy.
Complete answer:
We need to know that for incidents of hydrolysis; sodium acetate may be a sort of salt. When water is added to sodium acetate, the chemical bonds break down, causing it to separate into sodium ions and acetate ions. Then acetate ions in water then combine with hydrogen atoms to sort an acid called acetic acid.
Option A: the above reaction gives the product as propanoic acid and ethanol. But oxygen has 18 on its. So, this option is wrong.
CH3CH2COOC2H5H2OH2SO4CH3CH2COOH+C2H5OH
Option B: this reaction gives just one acid. But the reaction is carried and balanced properly. So, this option is wrong.
Option C: This reaction gives two acetic acid and formaldehyde and is balanced correctly. So this option is correct.
CH3CO−OCH2O−O=CCH3H2SO4H20CH3COOH+HCHO
Option D: This reaction gives propane-1,3-dioic acid and formaldehyde and is balanced perfectly. So, this option is correct.
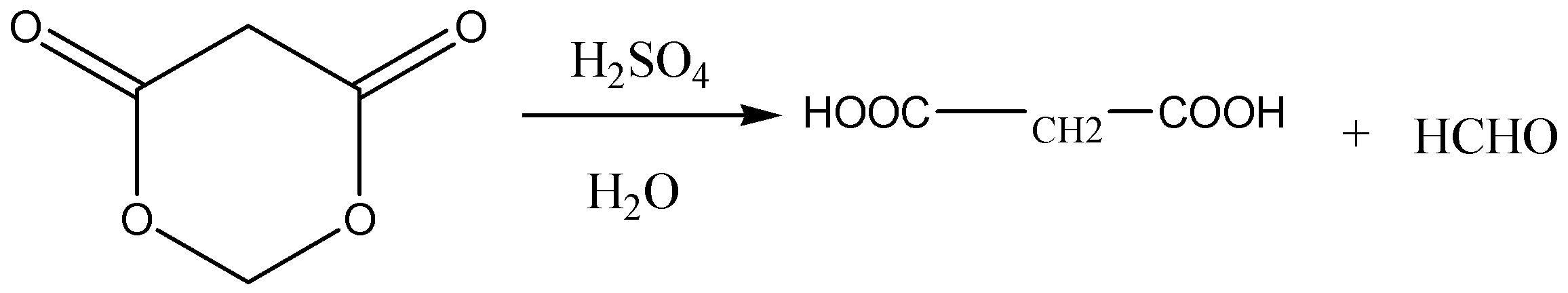
Therefore, the solution to this present question is option C and D.
Note:
We have to remember that the hydrolysis is widely used in industry to interrupt down chemicals into smaller fractions. For instance, when organophosphate ester undergoes a hydrolysis reaction then this hydrolysis aids within the production of insect killers and pesticide sprays.
