Question
Question: In which of the following, do all the C atoms not have the same hybridization? This question has m...
In which of the following, do all the C atoms not have the same hybridization?
This question has multiple correct options
A. 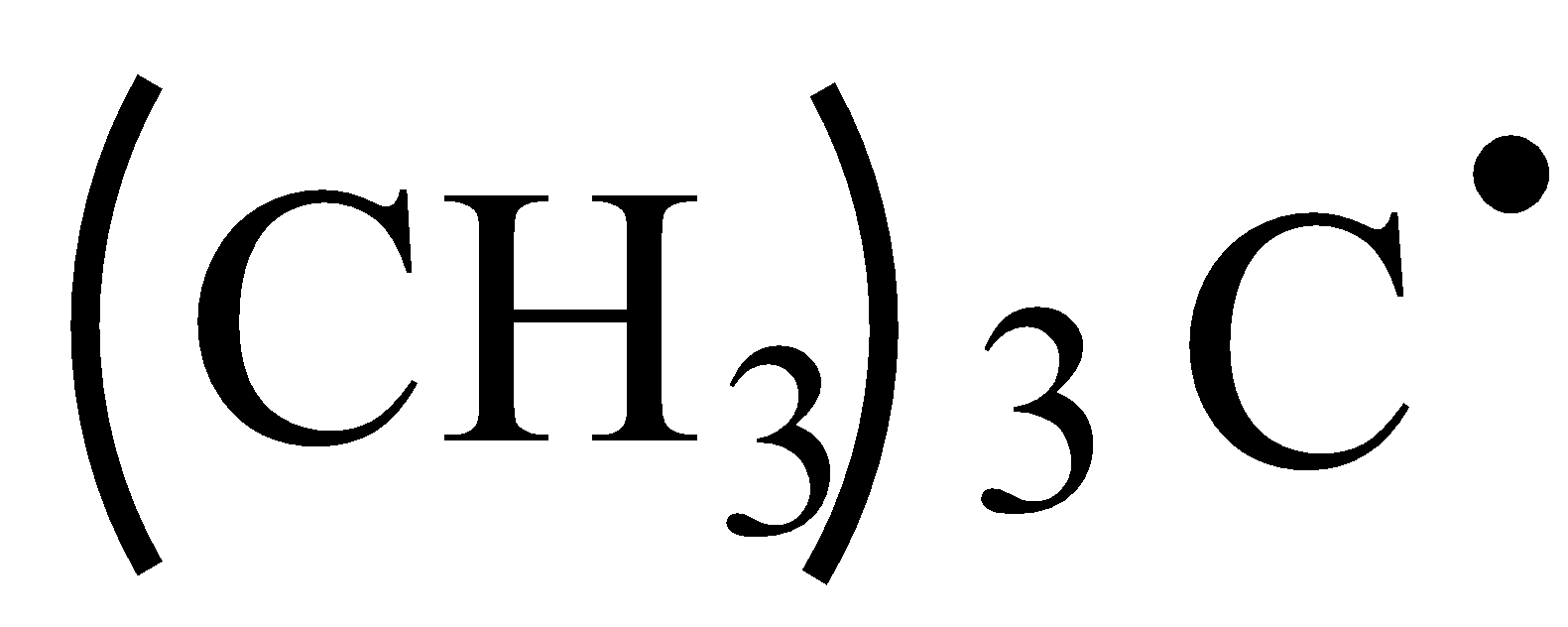
B. 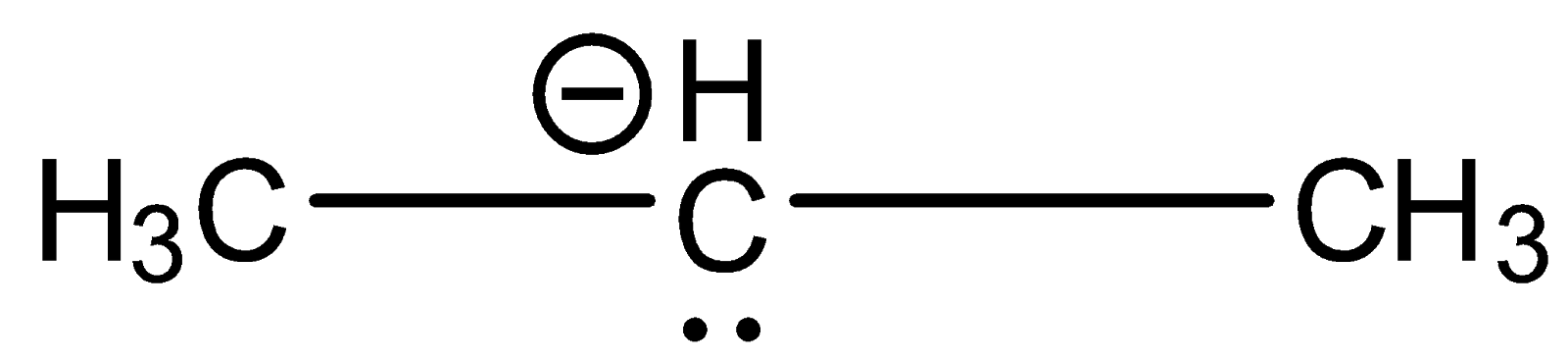
C. 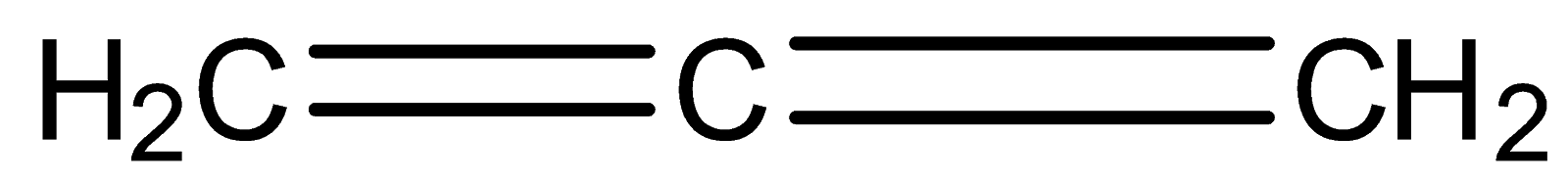
D. 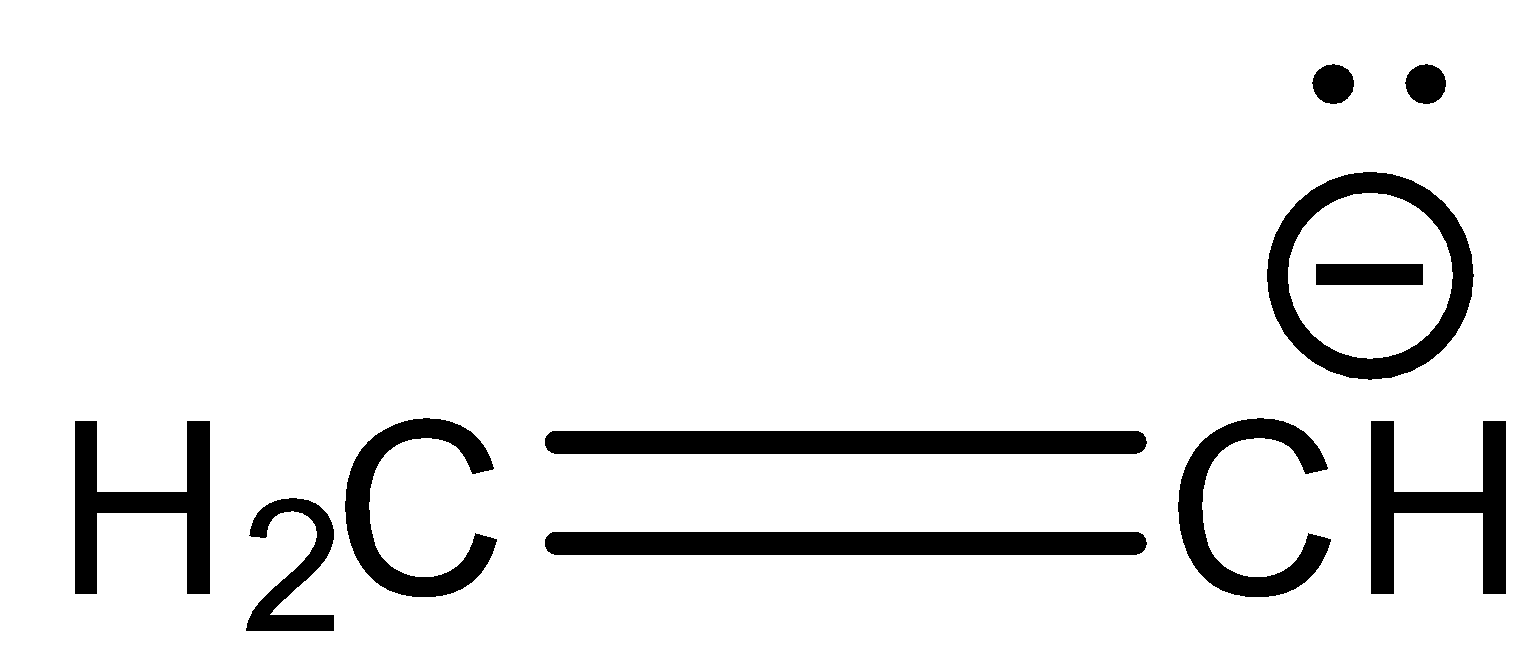
Solution
Hybridization is the phenomenon where orbitals of the same atom mixed with slight difference in energies so as to redistribute their energies and give new orbitals of equivalent energy and shape.
Complete step by step answer: Now, we discuss how to check hybridization of an atom.
If four electron groups surround an atom, the hybridization of the atom is sp3.
If three electron groups surround an atom, the hybridization of the atom is sp2.
If two electron groups surround an atom, the hybridization of the atom is sp.
Let’s identify the hybridization of the compound in option A.
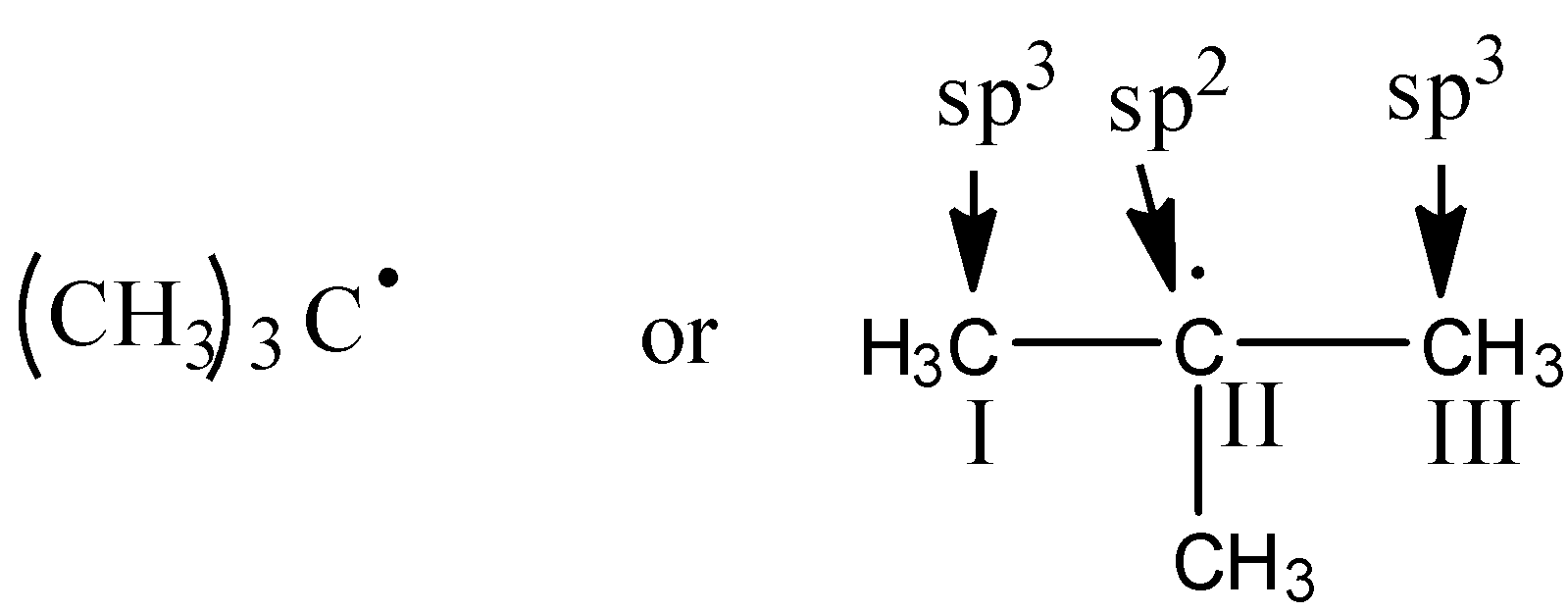
In the compound, 1st carbon is surrounded by four electron groups. So, hybridization of first carbon is sp3. 2nd carbon is surrounded by three electron groups, so hybridization is sp2 and 3rd carbon is surrounded by four electron groups, so, hybridization is sp3. Therefore, the hybridization of all carbon atoms in the compound is not the same.
Similarly we have to check the hybridization of the compound in option B.
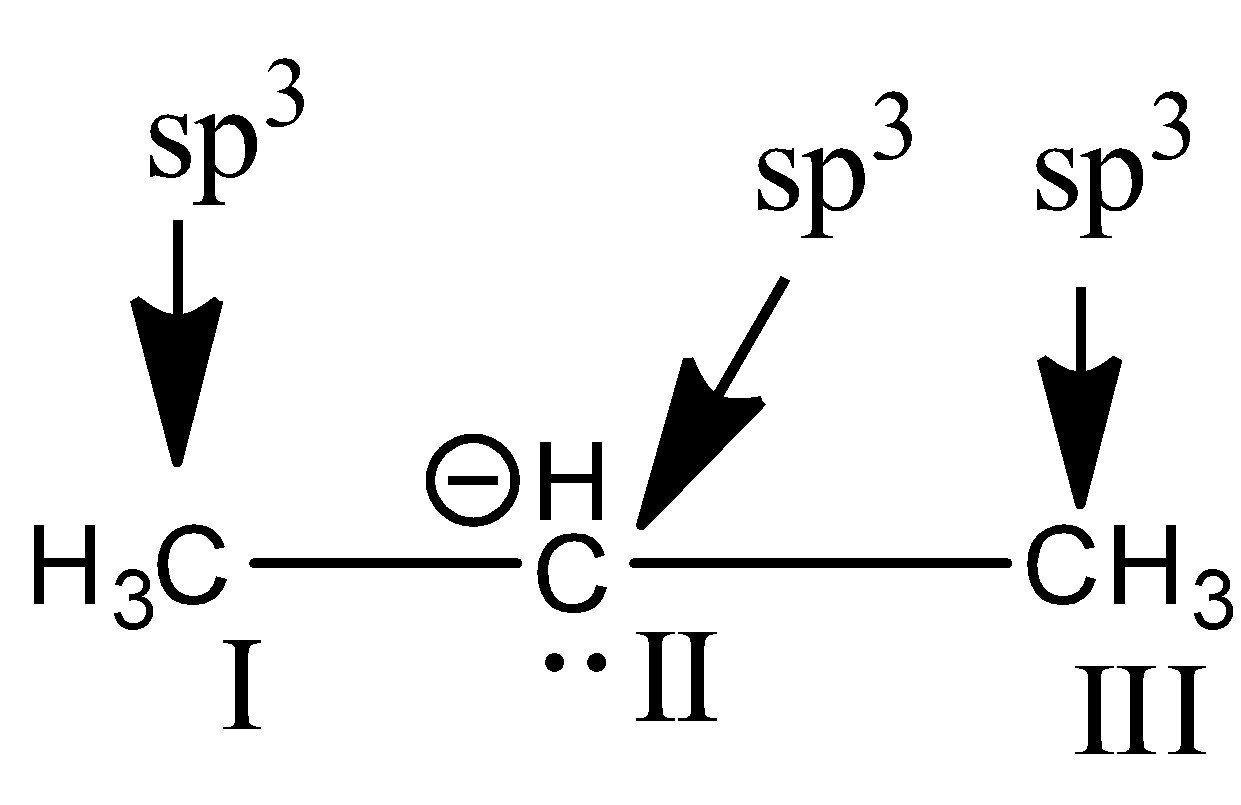
In the above compound, 1st carbon is surrounded by four electron groups. So, hybridization of first carbon is sp3. 2nd carbon is surrounded by four electron groups, so hybridization is sp3 and 3rd carbon is also surrounded by four electron groups, so, hybridization is sp3
Therefore, the hybridization of all carbon atoms in the compound is the same.
Similarly for the third compound we have to check hybridization.
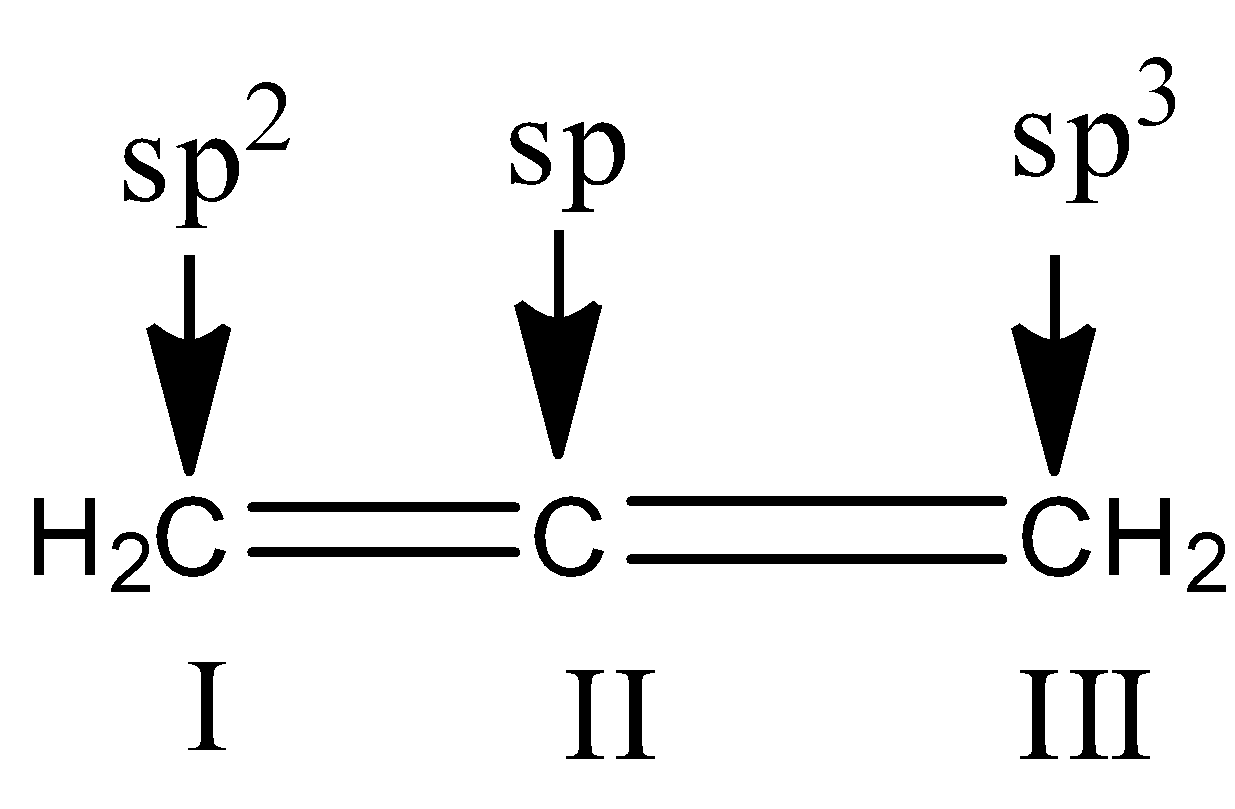
In the compound, 1st carbon is surrounded by three electron groups. So, hybridization of first carbon is sp2. 2nd carbon is surrounded by two electron groups, so hybridization is sp
and 3rd carbon is surrounded by three electron groups, so hybridization is sp2. Therefore, the hybridization of all carbon atoms in the compound is not the same.
Now, we check hybridization of the compound in option D.
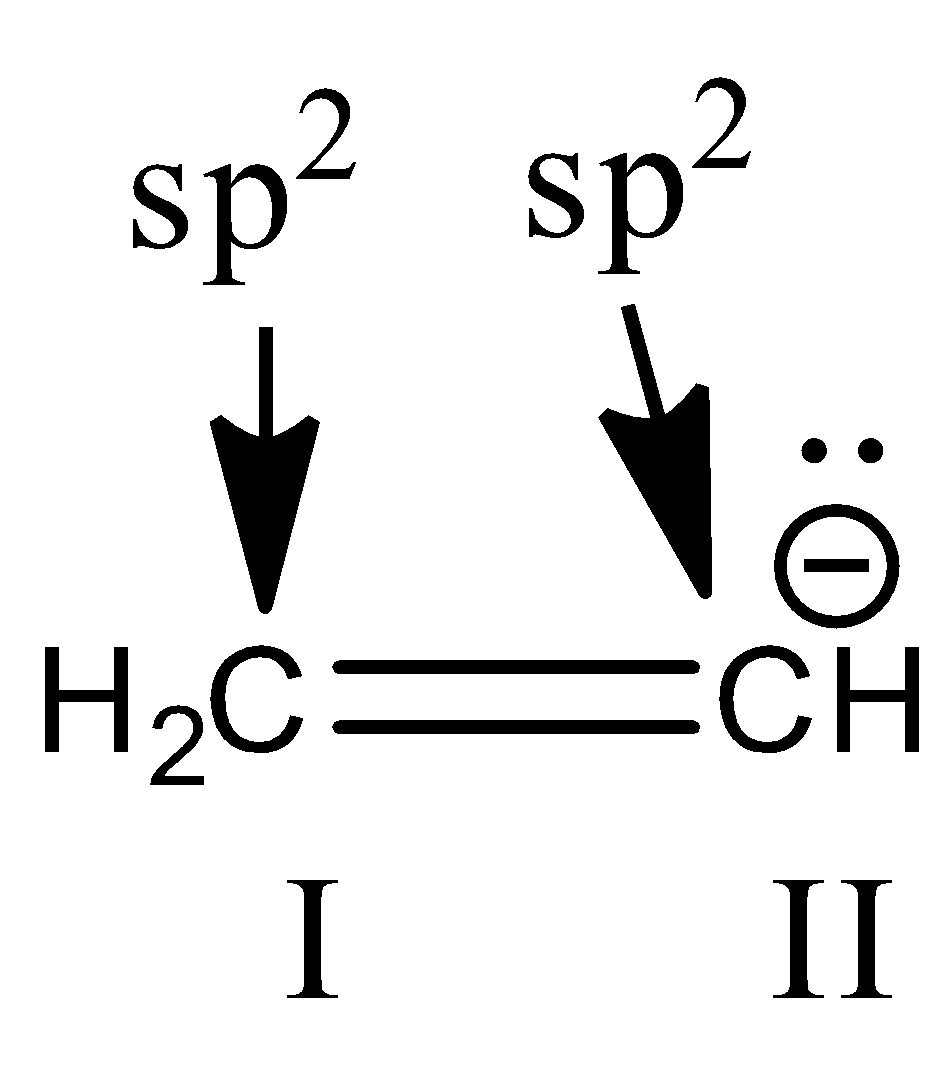
Here, 1st carbon is surrounded by three electron groups. So, hybridization of first carbon is sp2. 2nd carbon is also surrounded by three electron groups, so hybridization is sp2. Therefore, the hybridization of all carbon atoms in the compound is the same.
So, we find that the hybridization of all carbon atoms is not the same in the compound present in option A and C. Hence, option A and C is the correct answer.
Note: Always remember that lone pair is also considered as an electron group. An electron group can be a lone pair, bond pair or double or triple bond to an atom. The shape of molecules can be predicted considering the bond pair and lone pairs on the central atom.
