Question
Question: In which of the following diagrams, both Hund’s Rule and Pauli’s Exclusion principle are violated? ...
In which of the following diagrams, both Hund’s Rule and Pauli’s Exclusion principle are violated?
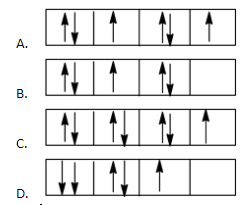
Solution
We know that there are certain rules for giving electronic configuration to an atom. These principles or rules are known as Aufbau Principle, Hund’s Rule of maximum multiplicity, and Pauli’s Exclusion principle. Aufbau principle tells us which orbital is to be filled whereas Hund’s Rule and Pauli’s Exclusion principle tells us how the electrons in the orbitals of the subshell are to be filled.
Complete step-by-step answer:
There are certain rules or principles for giving an electronic configuration to an atom or molecule. These principles or rules are known as Aufbau Principle, Hund’s Rule of maximum multiplicity, and Pauli’s Exclusion principle. Let's understand these principles.
Aufbau Principle: According to the Aufbau principle, orbitals are filled in order of increasing energy. This means the orbital with the lowest energy will be filled first.
1s 2s 2p 3s 3p 4s 3d 4p 5s 4d …….
It is important to note that the energy of an orbital is given by n+l rule. Lower the value of n+l, the lower is the energy level of an orbital. Here n is the Principal Quantum Number and l is the
Azimuthal Quantum Number. Principle number determines the size of an atom whereas Azimuthal Quantum Number gives 3D shape to the orbital. If n+l is the same for two orbitals, the orbital with a lower value of n if filled first.
Now the second rule is Hund’s Rule of maximum multiplicity, according to which the electrons should occupy all the degenerate orbitals first before doubling in the orbitals. Pauli’s Exclusion principle states that two electrons in the same orbital must have opposite spins.
From the given options, in the option, D both Hund’s Rule and Pauli’s Exclusion principle are violated.
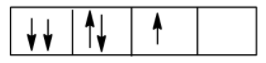
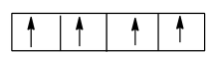
This is because according to Hund’s Rule of maximum multiplicity, the electrons should occupy all the degenerate orbitals first, therefore the correct order is
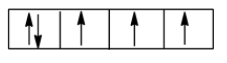
Pauli’s Exclusion principle states that two electrons in the same orbital must have opposite spins. So in the first orbital of the subshell, the electrons have the same spin. The correct form is
Hence the correct answer is option ‘D’.
Note: It is important to read the question carefully. It clearly asks the diagram which violates both Hund’s rule and Pauli’s Exclusion principle. In option A and B only Hund’s rule is violated but not Pauli’s Exclusion principle
