Question
Question: In which of the following compounds, the C-Cl bond ionisation shall give the most stable carbonium i...
In which of the following compounds, the C-Cl bond ionisation shall give the most stable carbonium ion?
[A] 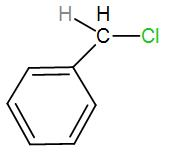
[B] 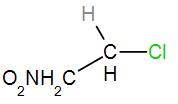
[C] 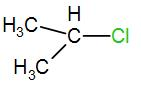
[D] 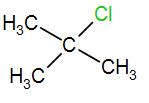
Solution
Stability of a carbocation depends on various factors. Here, the most stable carbocation will be the one which has a tertiary carbon atom. Here, the hyperconjugative effect will stabilize the carbocation even more than +R effect.
Complete answer:
Before answering this question, we have to know what a carbonium ion is and what affects its stability.
Carbonium ion is a pentavalent cation of carbon. We also know it as carbocation.
We know that a tertiary carbon atom is an atom to which three other carbon atoms are attached. Secondary and primary carbon atoms have two and one other carbon atoms attached to them respectively. They are also called 3∘,2∘ and 1∘ carbon atoms.
The stability of a carbocation depends upon several factors-
-Alkyl group stabilises carbocation by both +I effect and the hyper-conjugative effect. Bigger alkyl groups provide higher +I effect. The number of alpha-hydrogen in a compound gives the number of hyperconjugation structures. Higher the number of hyperconjugative structures, higher is the stability.Therefore, a tertiary carbocation is stable than a secondary carbocation which is stable than a primary carbocation.
- +R groups also stabilize a carbocation.
- Bulky groups attached to the carbocation stabilised it by preventing the carbon atom to return to the sp3 state.
- Some carbocations are also stabilised due to aromatization.
Now let us identify the type of carbon atoms in the given options to find out in which of them will have a stable carbocation on C-Cl bond ionisation.
In the first option, we have-
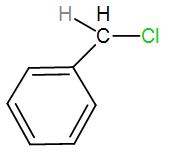
Here, bond ionisation of C-Cl will give a stable benzyl carbocation due to resonance. We can draw the resonance structures as-
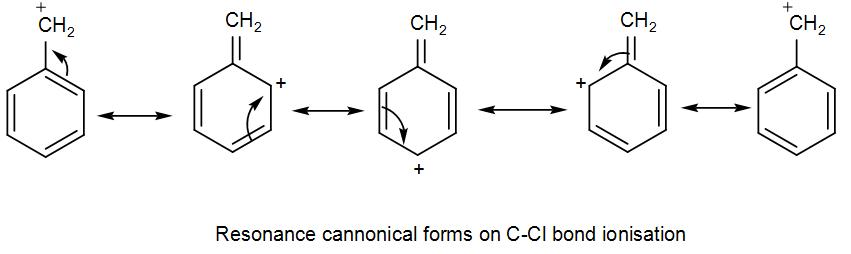
As we can see it has 4 resonance structures.
In the second option, we have
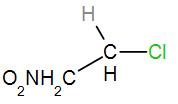
As we can see here that the central carbon atom is attached to only one more carbon atom which means it is a primary carbocation. Therefore, it will not form a stable carbocation.
In the next option, we have-
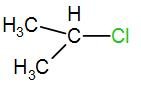
Here, the carbon is a secondary carbon. Therefore, it is somewhat stable also it has 6 alpha hydrogen atoms therefore it will have 6 hyperconjugative structures.
In the last option, we have
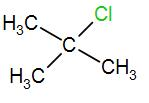
Here, we have a tertiary carbon atom, it will form a tertiary butyl carbocation and it has 9 alpha hydrogen atoms which means it has 9 hyperconjugative resonance structures. Therefore, it will also form a stable carbocation.
As we can see from the above discussion than both tertiary butyl and benzyl carbocation can be the most stable here but in the benzyl carbocation even though the positive charge is delocalised around the benzene ring, it always reacts on the side chain to preserve its aromaticity on the other hand the tertiary butyl carbocation has extra stability due to its planarity (by the weak donation of sigma bond electrons into the empty p-orbital of the cation if at least one C-H bond of each methyl group is parallel to one lobe of the empty p-orbital at one time. Here 3 such donations are possible by the three methyl groups thus making it more stable)
Planarity is an important factor for stability of carbocation. If it is not stable, it won’t be formed.
Therefore, the tertiary butyl carbocation will be more stable.
Therefore, the correct answer is option [D] .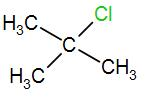
Note:
Here, the benzyl carbocation was less stable than the tertiary carbocation but an exceptionally strong carbocation is formed when three benzene rings are present to stabilize the same positive charge like in the case of triphenylmethyl cation, also known as trityl cation. It is used to form an ether with a primary alcohol group by SN1 reaction.
