Question
Question: In which of the following compounds does electron density on phenyl rings is maximum? A. 
B. 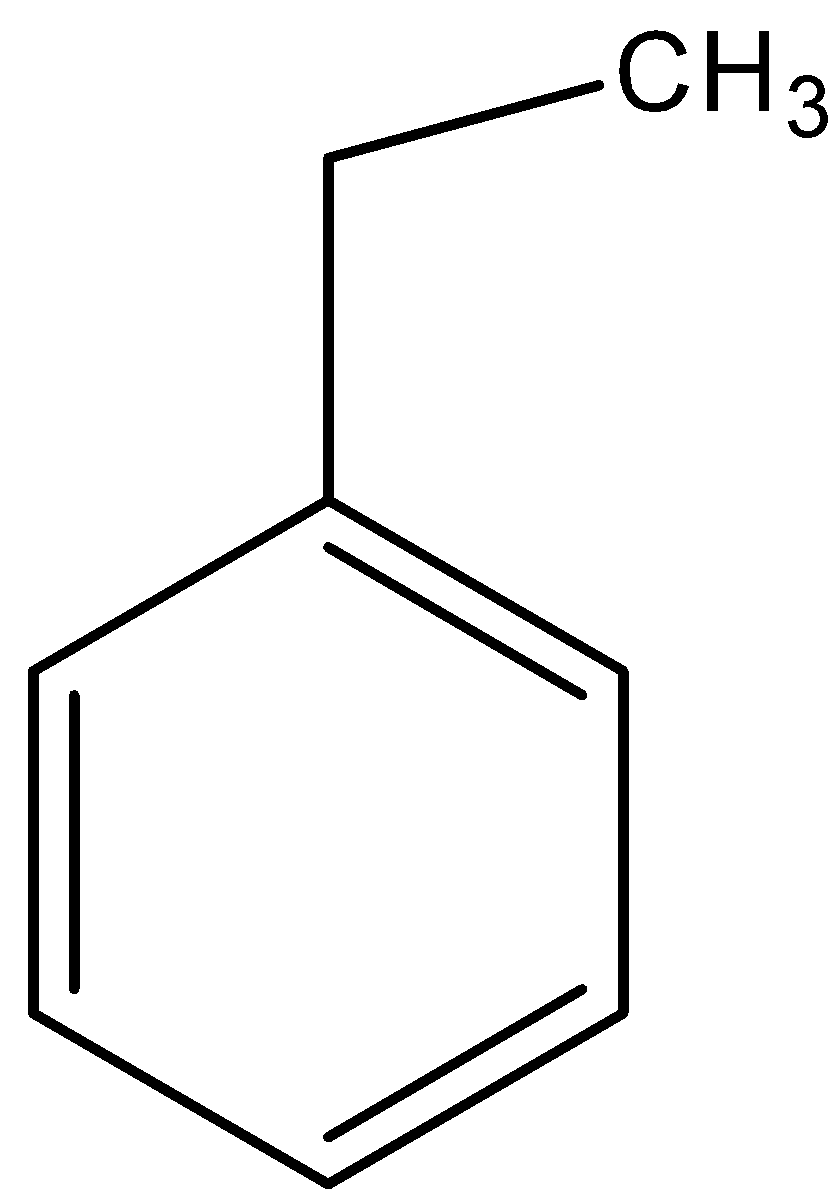
C. 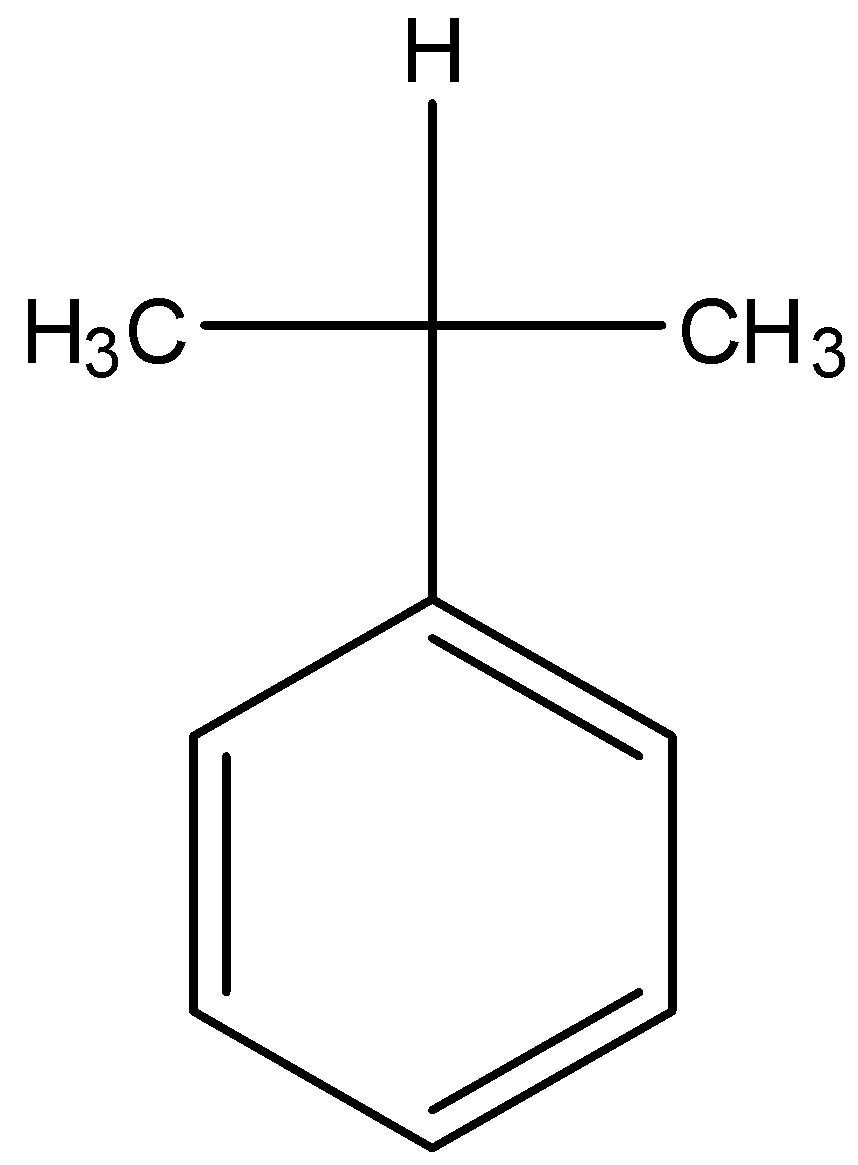
D. 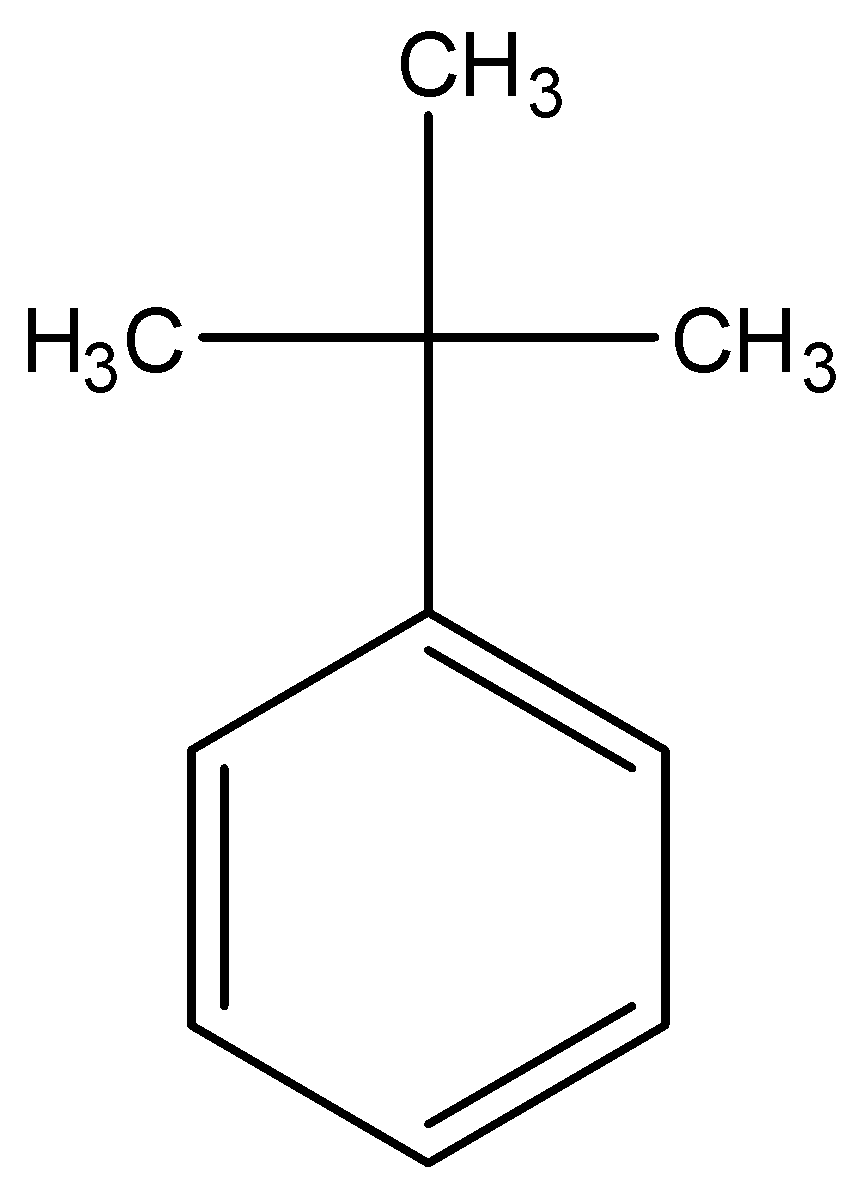
Solution
The electron density on a phenyl ring is going to depend on the number of alpha hydrogens which are present on the carbon which is attached to the phenyl ring.
- The alpha hydrogen is nothing but the hydrogen which is present on the alpha carbon and it is shown the below picture.
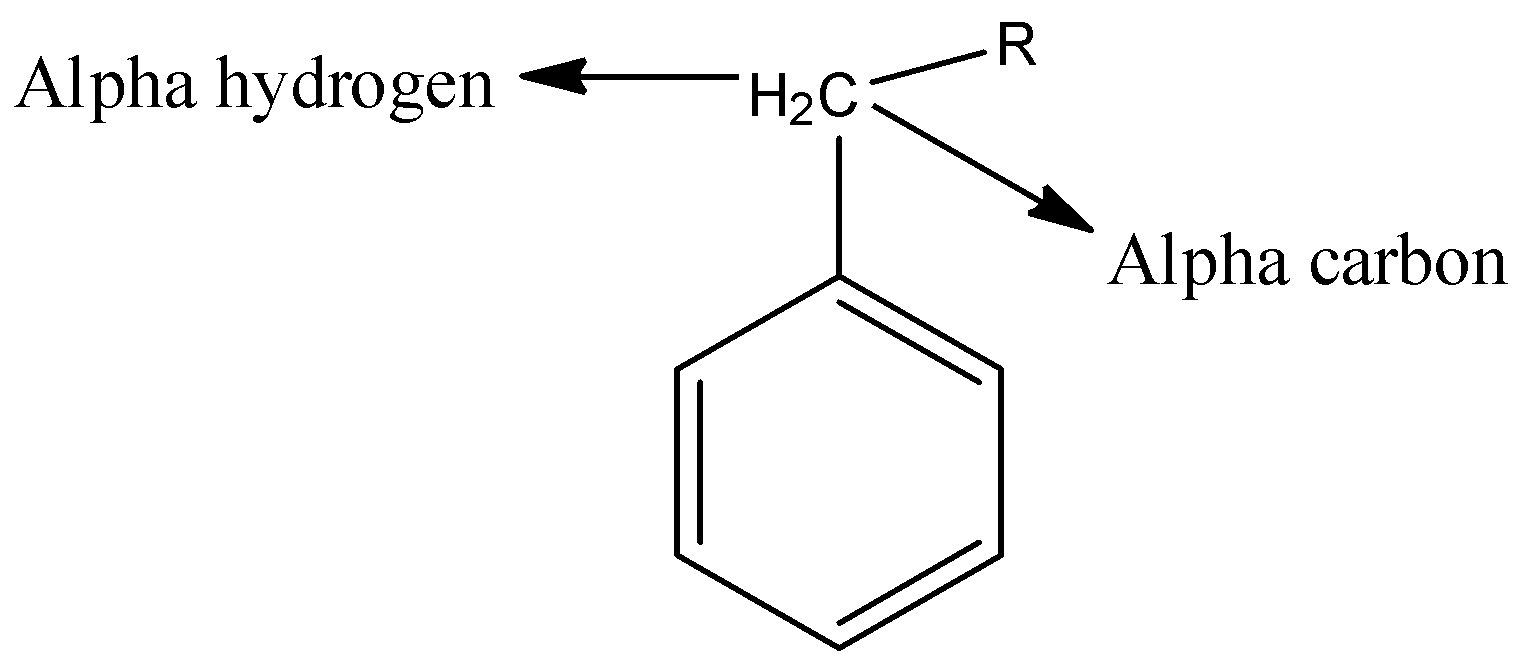
Complete Solution :
- In the question it is given which compound has high electron density on the phenyl ring among the given options.
- To know about the electron density on the phenyl ring we should know about the number of alpha hydrogens which are present in the given options.
- The alpha hydrogens in the phenyl derivatives cause the formation of hyperconjugation structures due to this reason the electron density will be high in the molecules which have large numbers of alpha hydrogens.
- Coming to given options, option A, there are three alpha hydrogens.
- Coming to option B, there are two alpha hydrogens.
- Coming to option C, there is only one alpha hydrogen.
- Coming to option D, there are no alpha hydrogens.
- Therefore option A only has a high number of alpha hydrogens (three) in its structure.
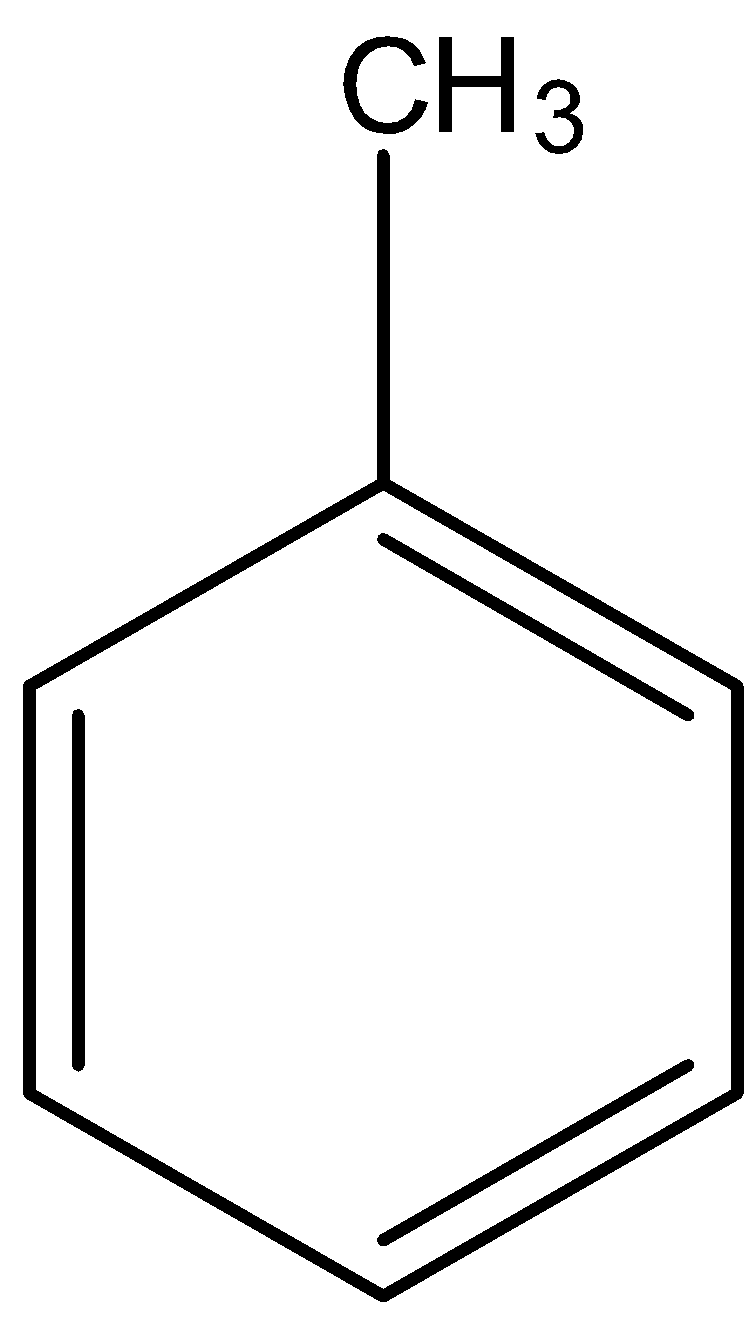
So, the correct answer is “Option A”.
Note: The alpha hydrogens are the reason behind the formation of the hyper conjugative structures. If the phenyl derivatives have a large number of hyper conjugative structures means more electron density is present on the phenyl ring.
