Question
Question: In which of the following compounds delocalisation is not possible A.1,4-pentadiene B.1,3-butad...
In which of the following compounds delocalisation is not possible
A.1,4-pentadiene
B.1,3-butadiene
C.1,3,5-hexatriene
D.Benzene
Solution
The phenomenon of hyperconjugation is the property of compounds having alternate double and single bonds. These alternate double and single bonds consist of delocalization of electrons. Delocalization means the movement of electrons to acquire stable positions in the molecule.
Complete answer:
The phenomena of delocalization is the movement of electrons that are not in a bonded state inside a molecule that results in a highly stable molecule. The electrons involved in the delocalization are called delocalized electrons.
The delocalized electrons do not remain in one place and tend to rotate, this provides the molecule with extra stability.
The double bonds contain the pi bonds that are made of electrons which are loosely held, this creates the loosely held electrons to move and hence they become delocalized. The delocalization results in the higher stabilization of energy in the molecule.
With this basic knowledge of delocalization, let us get into the options.
1,4-pentadiene
CH2=CH−CH2−CH=CH2
There is no alternate double and single bond. Hence, this compound cannot be delocalized.
1,3-butadiene
CH2=CH−CH=CH2
There is alternate double and single bond, hence the compound can be delocalized.
1,3,5-hexatriene
CH2=CH−CH=CH−CH=CH2
This compound also contains alternate double and single bond, hence can be delocalized.
Benzene
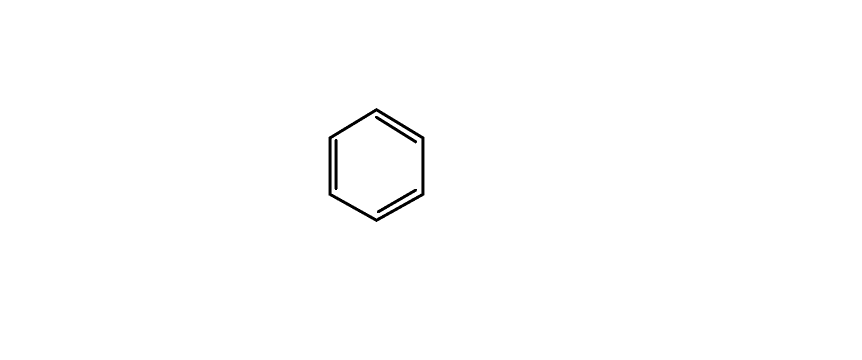
Benzene is the universal example for delocalization of electrons. It contains alternate double and single bonds and hence can be delocalized.
So, 1,4-pentadiene is the only compound that cannot be delocalized.
Hence the correct answer is option A, 1,4-pentadiene.
Note:
The hyperconjugation and inductive effect are quite opposite. The inductive effect is through sigma bond and the hyperconjugation is through the charge transfer.
