Question
Question: In which of the following compounds, cross conjugation are present? 
(a)- x, y
(b)- y, z, w
(c)- x, y, z, w
(d)- Only z
Solution
Cross-conjugation is a type of conjugation in which there are three pi-bond systems and these are in conjugation. The conjugation is in such a way that the first pi-system is in conjugation with the second pi-system and the second pi-system is in conjugation with the third pi-system but the first and third pi-systems are not in conjugation with each other.
Complete Solution :
The conjugation system of pi-bonds in compounds means there are alternate single and double bonds in the compound. 1, 3-Butadiene is an example of a conjugated system. There are two bonds and one single bond in 1, 3-Butadiene. The formula is given:
CH2=CH−CH=CH2
- Cross-conjugation is a type of conjugation in which there are three pi-bond systems and these are in conjugation. The conjugation is in such a way that the first pi-system is in conjugation with the second pi-system and the second pi-system is in conjugation with the third pi-system but the first and third pi-systems are not in conjugation with each other.
So, from the given compounds in the question:
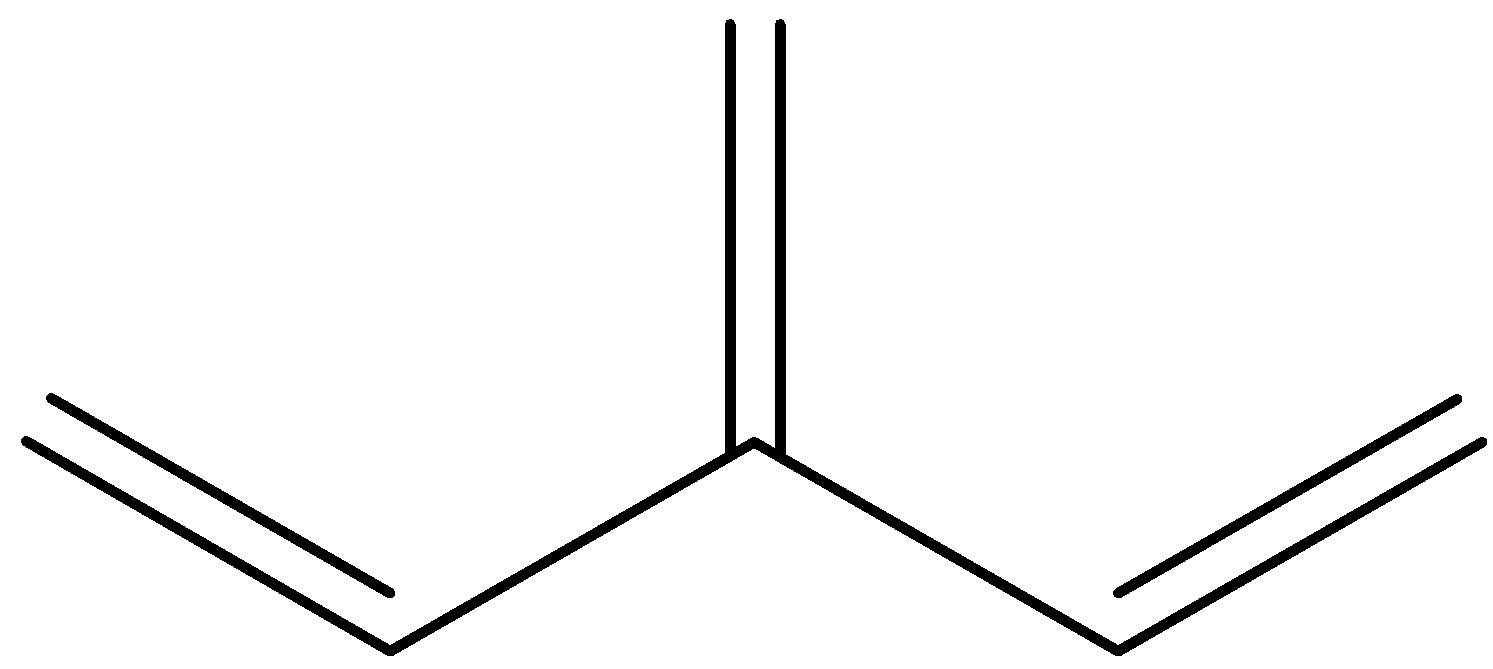
Is an example of a cross-conjugation system. Let us give numbers to all the pi-bond in the system:
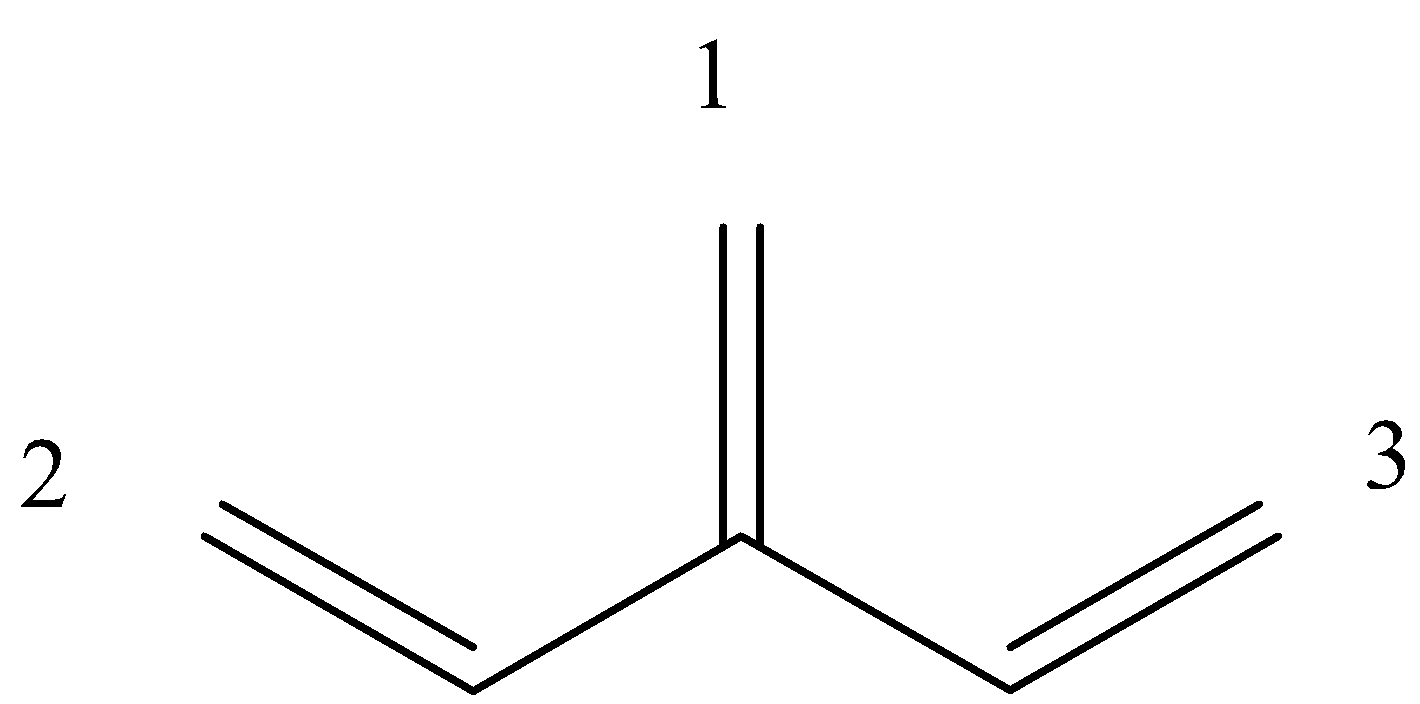
So, we can see that 1 and 2 are in conjugation, and 1 and 3 are in conjugation, but 2 and 3 are not in conjugation because there are 2 single bonds between them.
So, the correct answer is “Option D”.
Note: It must be noted that only two systems must be in the main chain and the third system must be in the side chain. Some other examples of cross-conjugation are benzophenone, divinyl ketones, fullerene, etc.
