Question
Question: In which of the following complex, cis-trans isomerism is possible? A. \({[Pt{\left( {en} \right)_...
In which of the following complex, cis-trans isomerism is possible?
A. [Pt(en)2Cl2]2+
B. [Cr(en)2(Ox)]+
C. [Pt(en)Cl2]
D. [Pt(en)2]2+
Solution
We have to know that if there is restricted rotation in a molecule there arise geometric isomerisms. Geometric isomers are known as cis- trans isomerism.
Complete step by step answer:
We know that the compounds with the same molecular formula but differ in the arrangement of the atom are said to be isomers. We can say stereoisomers are the isomers that have the same molecular formula but differ in spatial arrangement of atoms. Stereoisomers can be subdivided into two groups,
1.Optical isomers
2.Geometrical isomers
If there is restricted rotation in a molecule there arise geometric isomerisms. Geometric isomers are known as cis- trans isomerism.
1.If the two atoms locked in same side of the molecule then it is called as cis isomers.
2.If the two atoms are locked on opposite sides of the molecule then it is called trans isomers.
We can draw the structure of [Pt(en)2Cl2]2+ are,
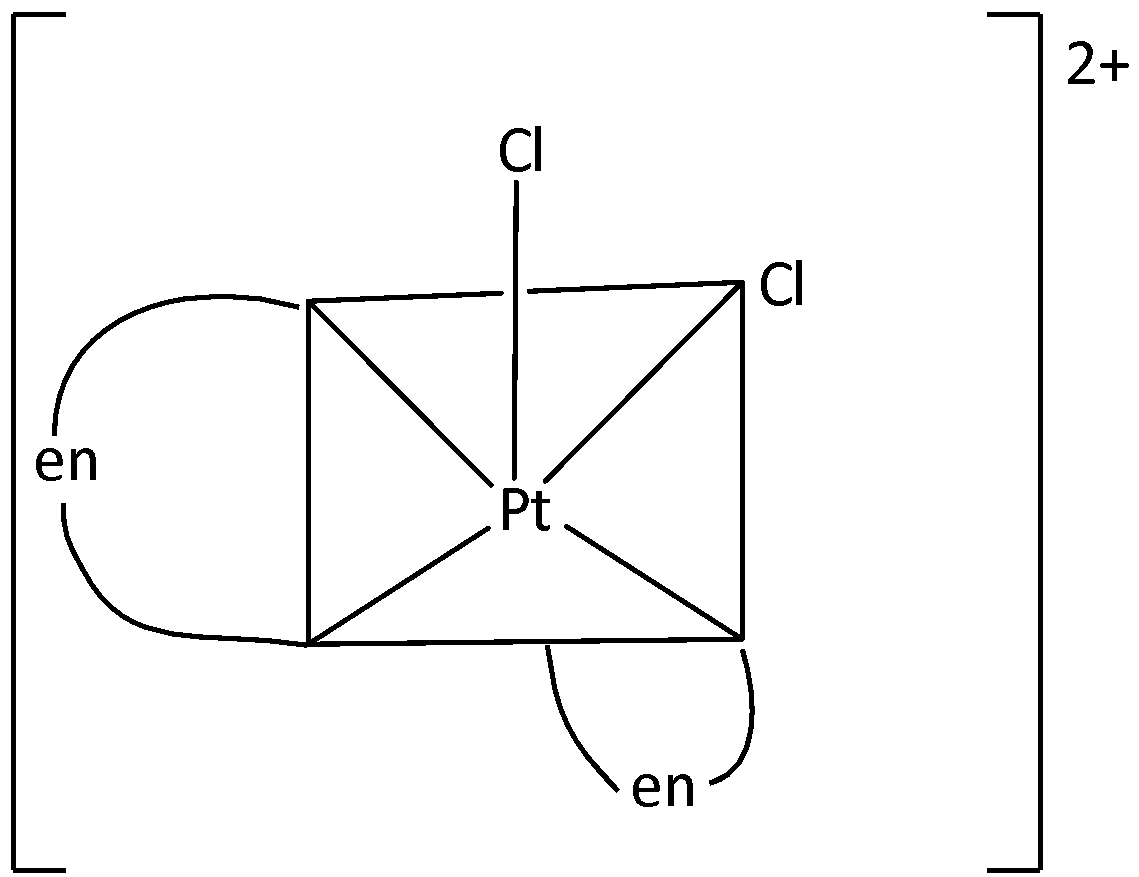
There is a possibility for a restricted rotation in the molecule. Hence, it could rise to geometrical isomers. The two possible geometrical isomers
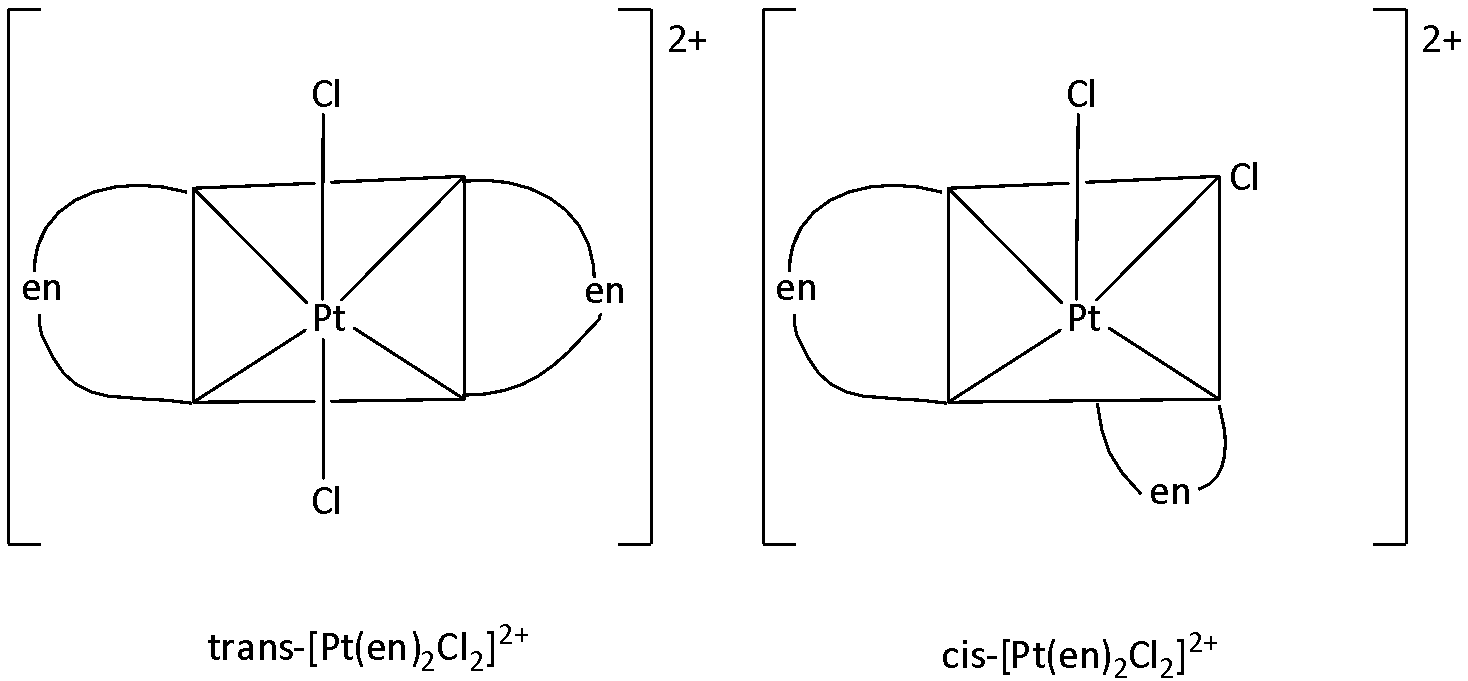
Therefore, Option (A) is correct.
We can draw the structure of [Cr(en)2(Ox)]+ as
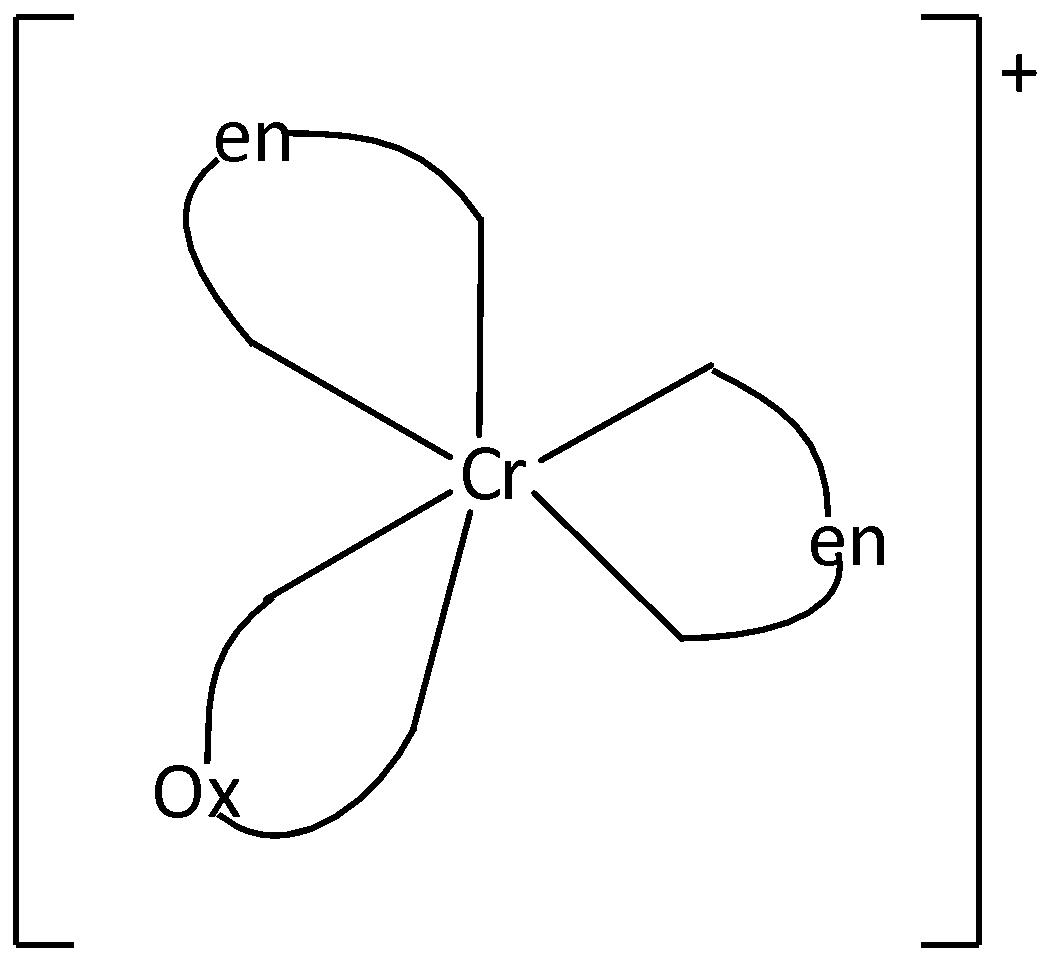
From the structure, it is understood that there cannot be any restricted rotation in the molecule. Cis-Trans isomerism is not possible
Therefore, Option (B) is incorrect.
We can draw the structure of [Pt(en)Cl2] is
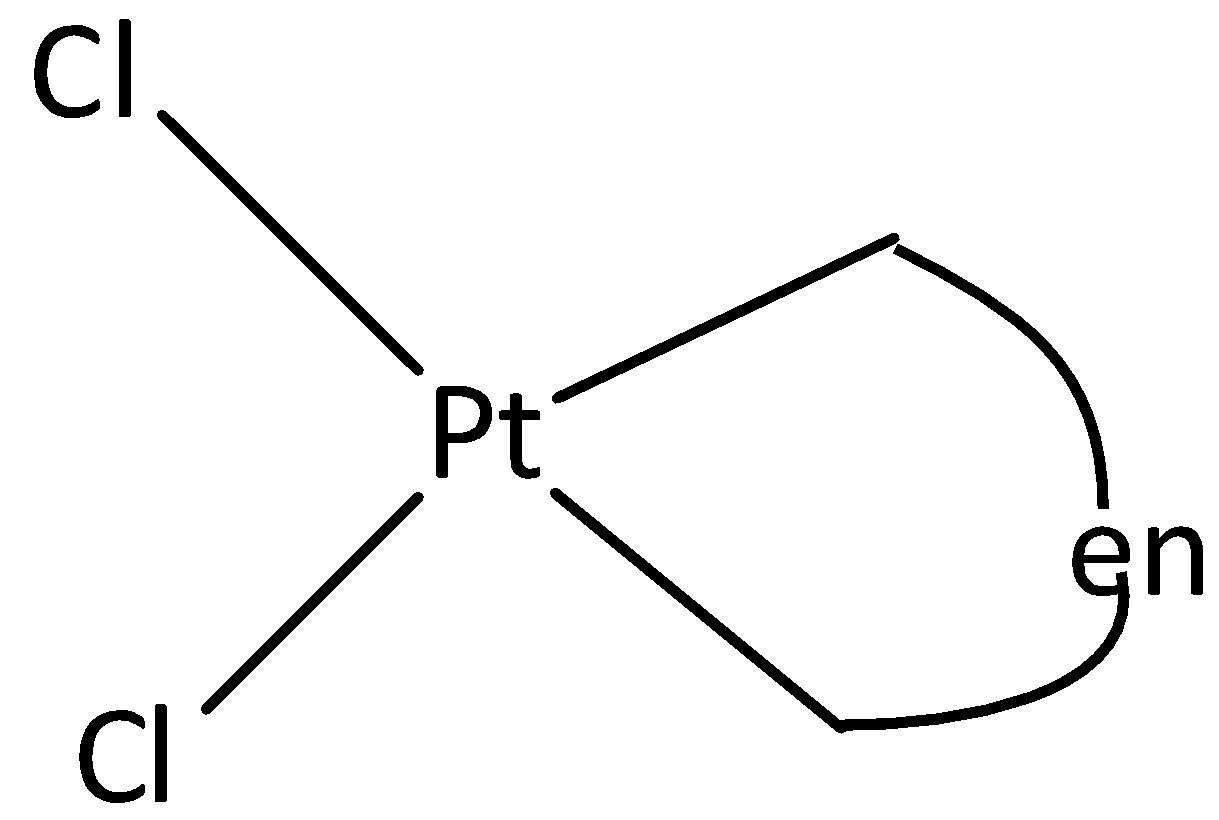
From the structure, it is understood that there cannot be any restricted rotation in the molecule. Cis-Trans isomerism is not possible .
Therefore, Option (C) is incorrect.
We can draw the structure of [Pt(en)2]2+ as
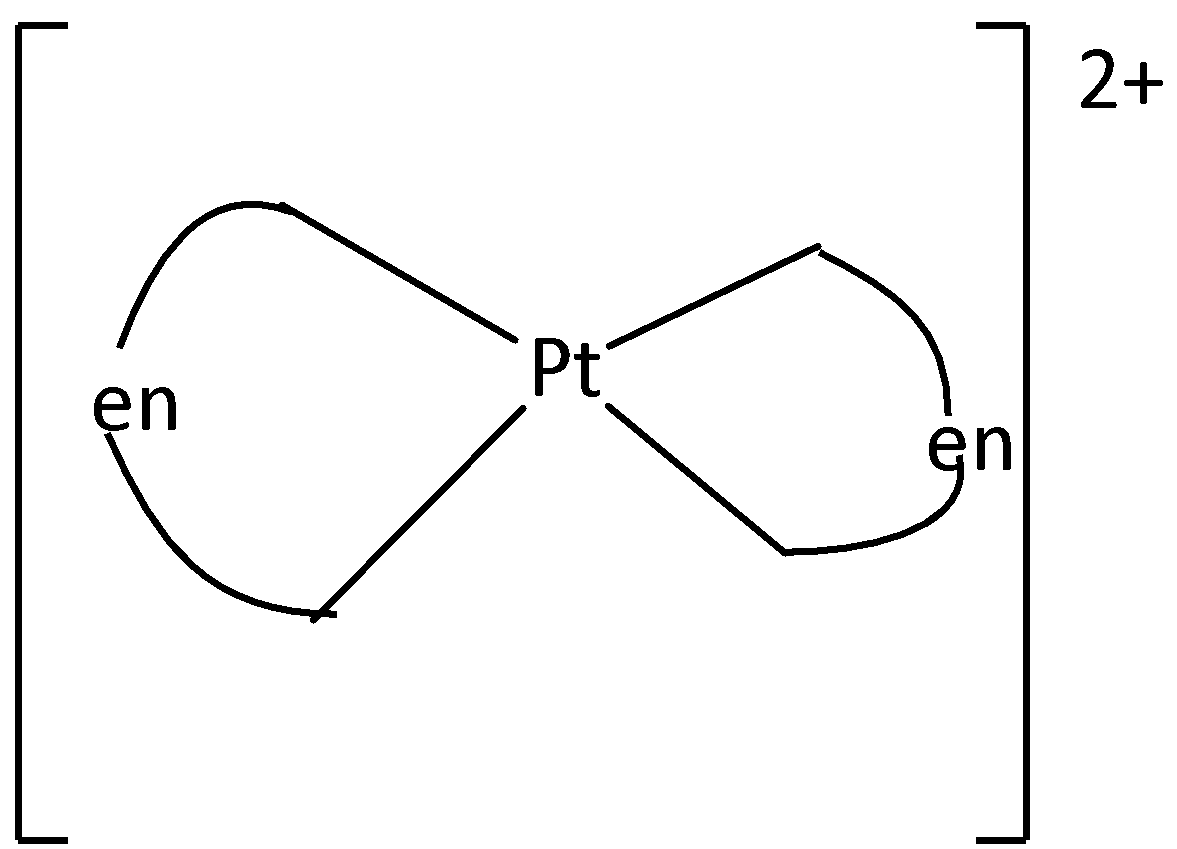
From the structure, it is understood that there cannot be any restricted rotation in the molecule. Cis-Trans isomerism is not possible .
Therefore, Option (D) is incorrect.
So, the correct answer is Option A.
Note:
We have to know that the other possible isomers in coordination compounds are,
Hydrate Isomers:
Hydrate isomers differ by the exchange of molecules with another ligand in the coordination sphere. It can be distinguished by the stoichiometry of reactions in which the ion is exchanged with water.
Linkage isomers:
Linkage isomerism takes place with ambidentate ligands that have the capacity of coordinating in more than one way.
Example:
The only difference is the atoms bind to the central ion. The ligand must have more than one donor atom but bind to ions in only one place.
Coordination Isomers:
Coordination isomerism arises in compounds containing complex anionic and cationic parts. So, there are two complex compounds linked together, one contains a negative charge and the other contains a positive charge. In coordination isomers, exchange one or more ligands happens between the anion and cations of the complex.
Ionization Isomers:
Ionization isomers occur when a ligand bound to the metal center exchanges places with an anion or neutral molecule that was previously outside the coordination complex.
