Question
Question: In which of the compounds, cross conjugation is present? 
A.X,Y
B.Y,Z,W
C. X,Y,Z,W
D. onlyZ
Solution
Conjugation is the overlap of one p-orbital with another across an intervening sigma bond. In larger atoms d-orbitals can be involved. The compounds in which the bonds are conjugated (alternating multiple and single bonds) are slightly more stable than those in which they are isolated.
Complete step by step answer:
Cross conjugation is a special type of conjugation in a molecule. When in a set of three Pi bonds, only two Pi bonds interact with each other by conjugation and the third one is excluded from interaction.
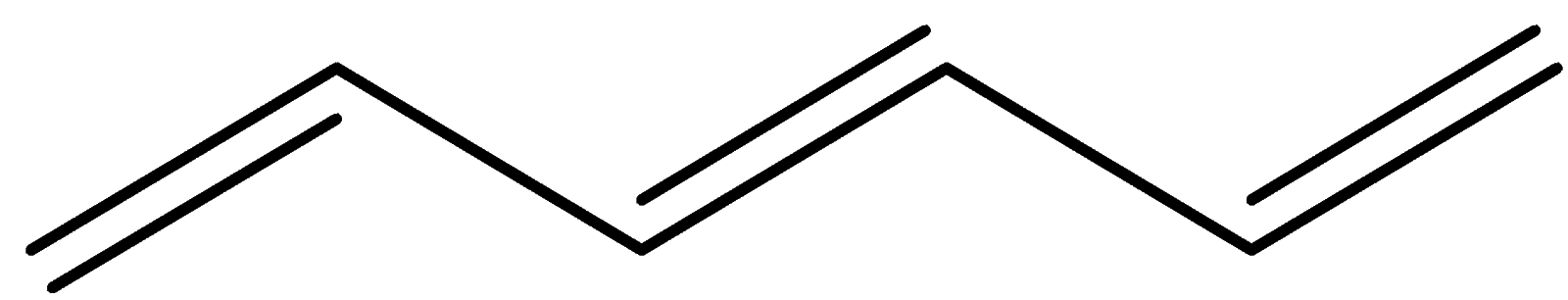
In this structure, there’s a double bond then a sigma bond and then a double bond and then a sigma bond. This is called conjugation. Here we can see overlap of one p-orbital with another p-orbital and also the sigma bond is intervening between the two.
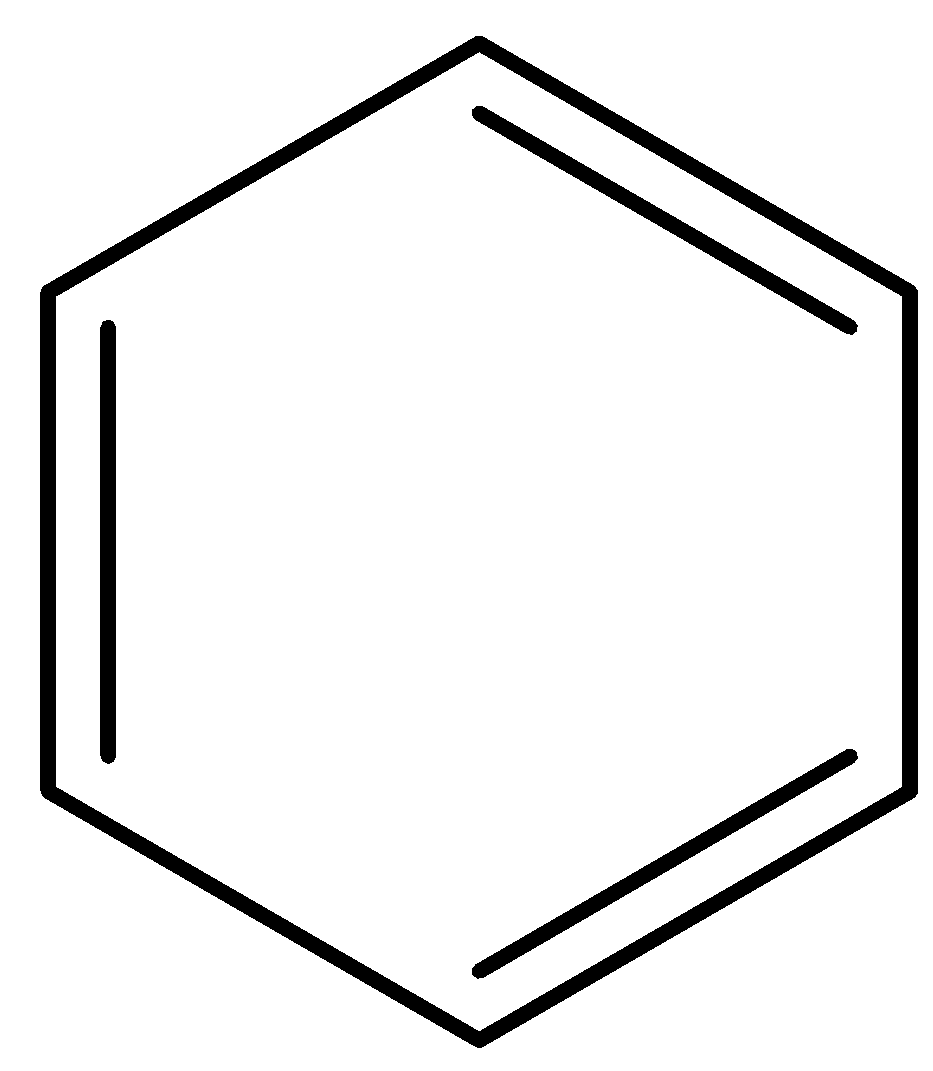
Even in this structure there’s conjugation as sigma bonds are intervening between the double bonds.
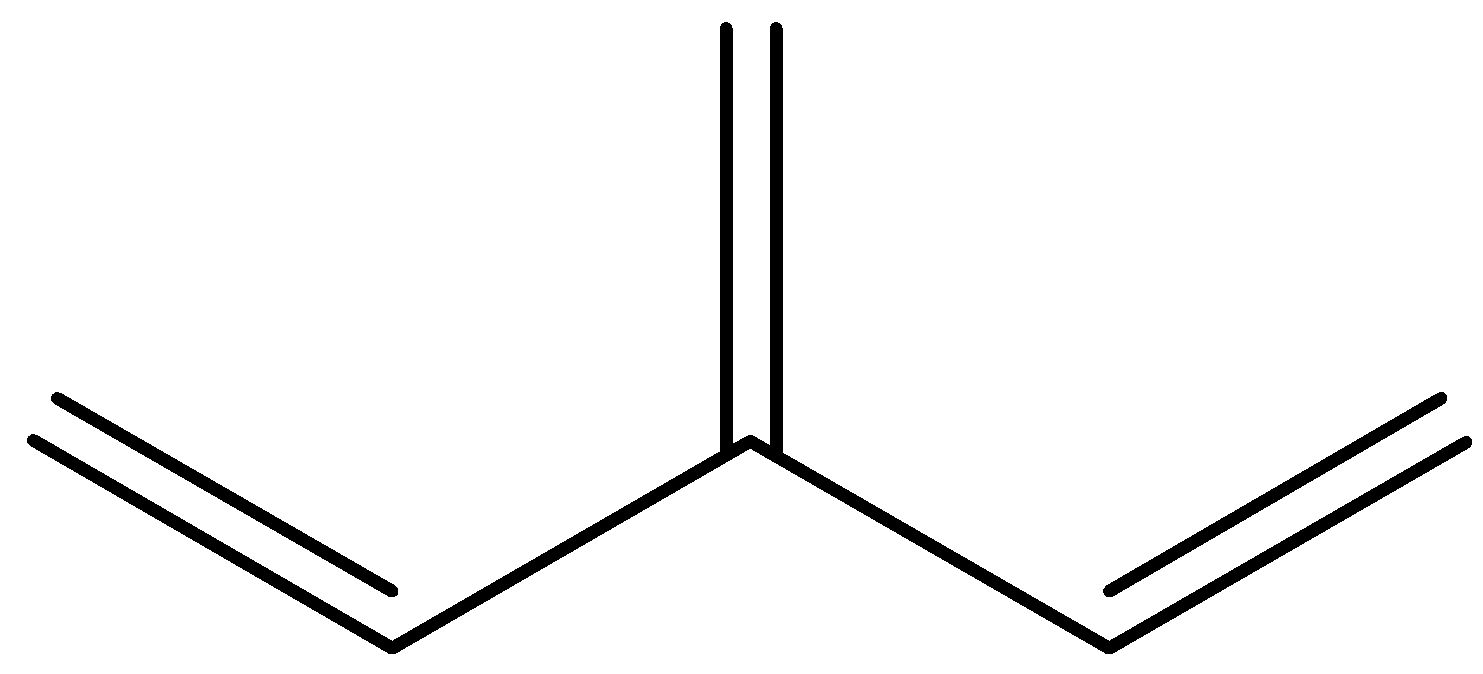
Here, out of a set of three Pi bonds only two interact with each other by conjugation, while the third one is excluded from interaction. Here we have a double bonded unit single bonded to one of the middle atoms of another conjugated chain. In other words, one of the double bonds branches off rather than continuing consecutively. Hence, a main chain is conjugated and part of that same main chain is conjugated with the side group, but all parts are not conjugated together. So, this structure is cross conjugated.
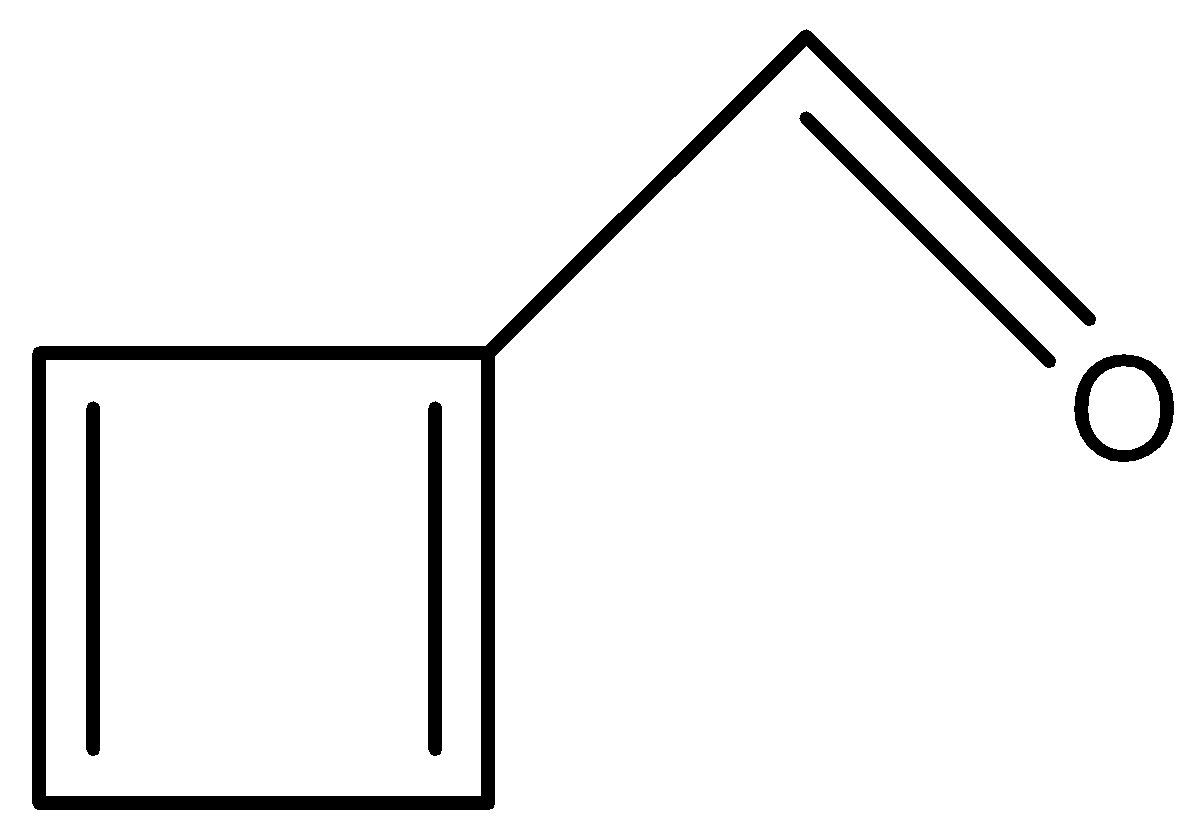
In this option, we see conjugation again.
Hence the correct answer is D.
Note: Cross conjugation has an important impact on reactivity and molecular electronic transition.Examples are Benzophenone, Divinyl ketone, p-quinone.Due to the cross conjugation, the extent of resonance decreases slightly.
