Question
Question: In which case, the reaction is most exothermic with \({H_2}Ni\). A.
B.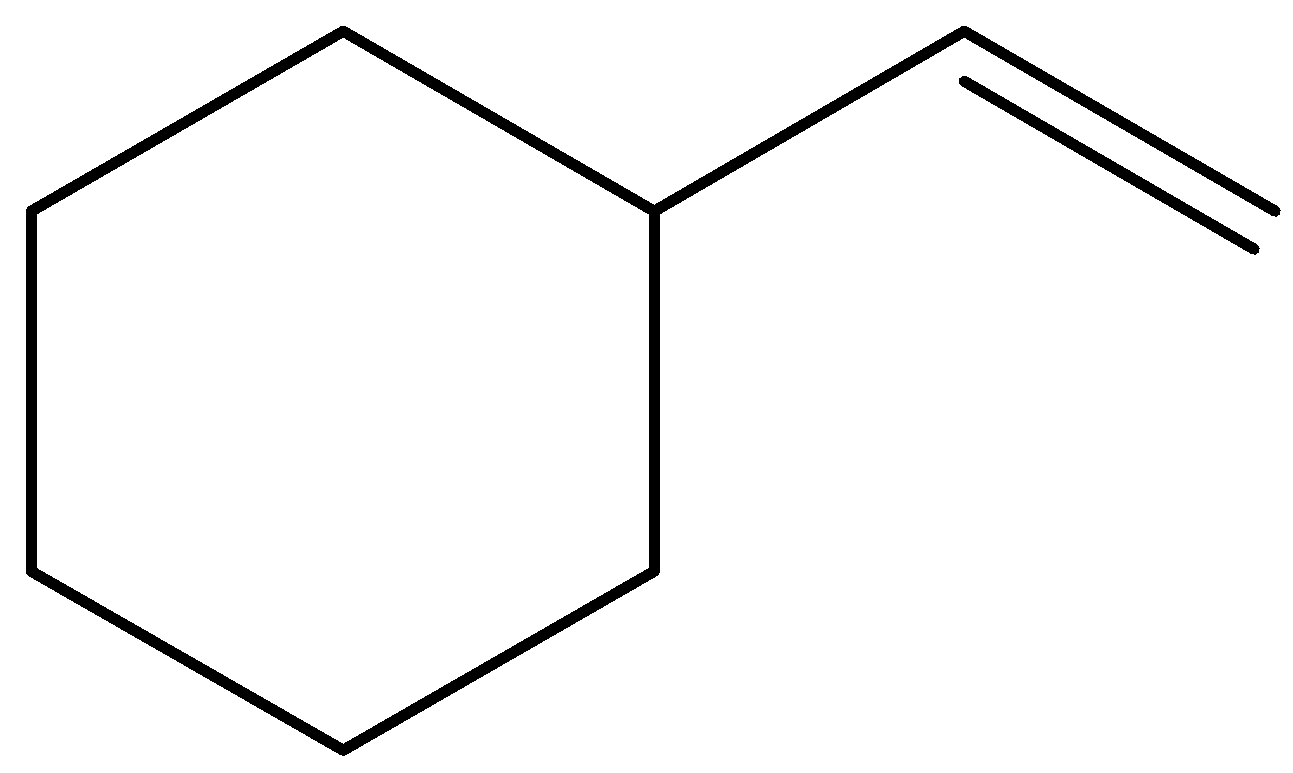
C.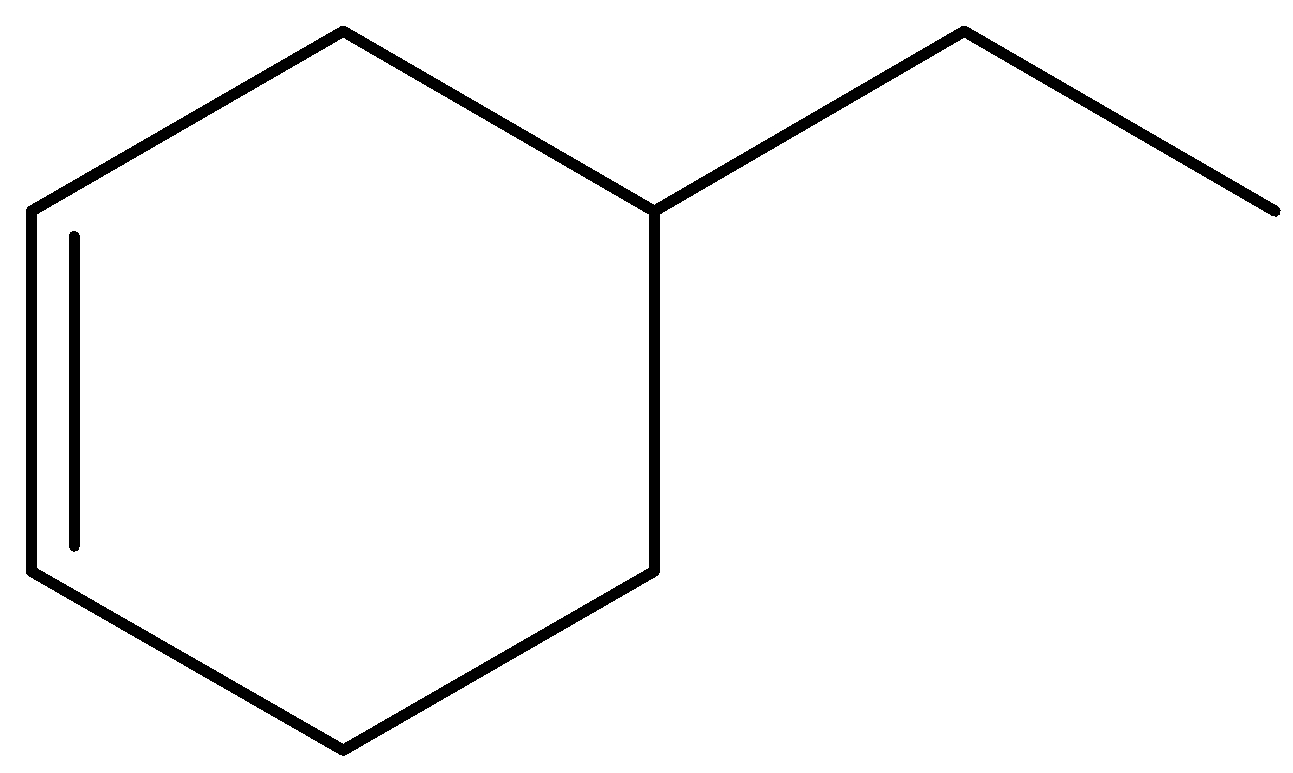
D.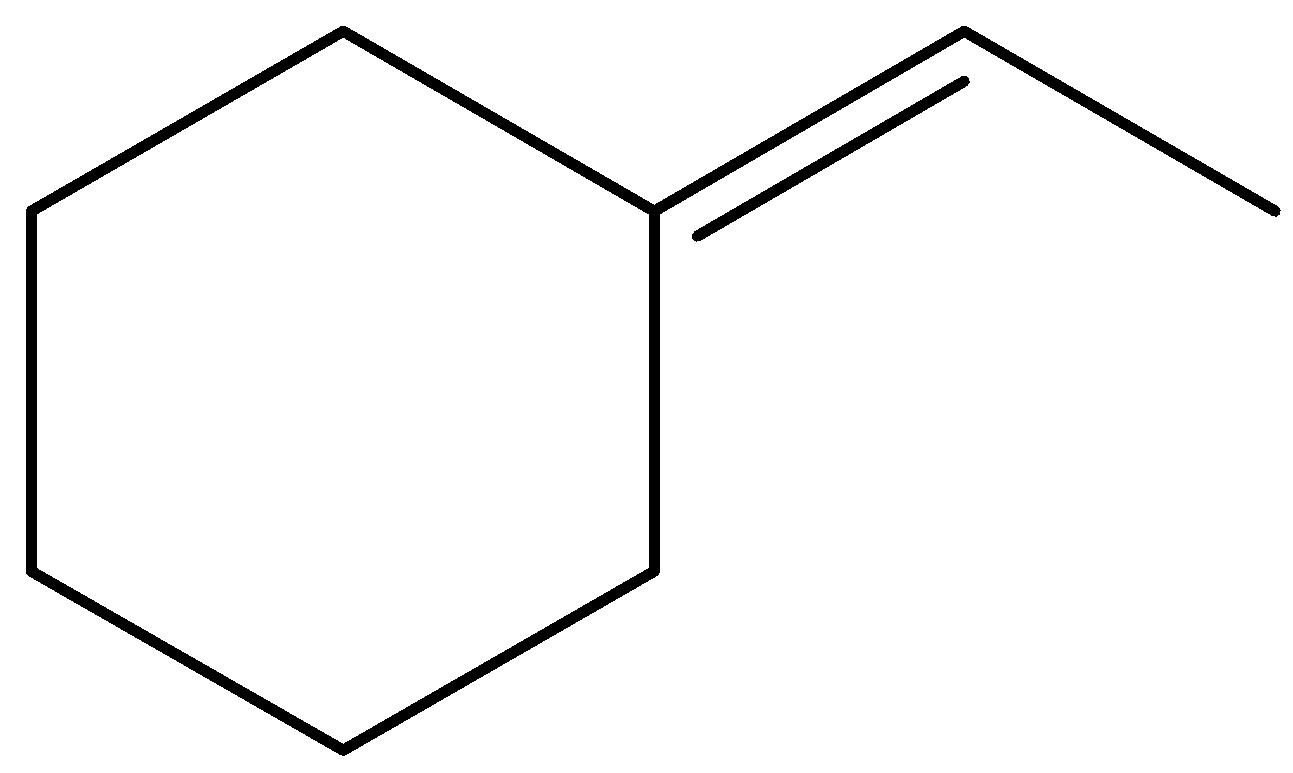
Solution
We have to know that the amount of heat energy liberated is dependent on the energy of the initial molecule. If the energy of the molecule is higher, then the stability of the molecule is lesser.
Complete step by step answer:
The energy difference between the initial and final molecules is the heat of the reaction.
We know that those reactions that release heat energy to the surroundings are called exothermic reactions. The formation of chemical bonds causes the releases of heat energy.
We could get the product as ethyl cyclohexane when we hydrogenate all the four of the molecules. The molecule would have lesser stability, if the energy released by the molecule would be higher.
If the number of alkyl groups attached is greater, then the stability of the alkenes is greater.
Increase in substitution, decreases the heat of the hydrogenation. Increase in substitution, increases the stability of the alkene. Most substituted alkenes are generally favored when compared to less substituted alkenes.
Tetrasubstituted alkenes are highly stable / favourable because they contain greater number of alkyl groups and monosubstituted / unsubstituted alkenes are less stable / favourable because the substitutions are less in these alkenes.
In the given options, molecules (A) and (D) are trisubstituted, molecule (C) is disubstituted, and molecule (B) is monosubstituted.
The least stable molecule is structure (B). It could contain the highest energy among the given molecules, therefore could be highly exothermic.
The molecule (B) is,
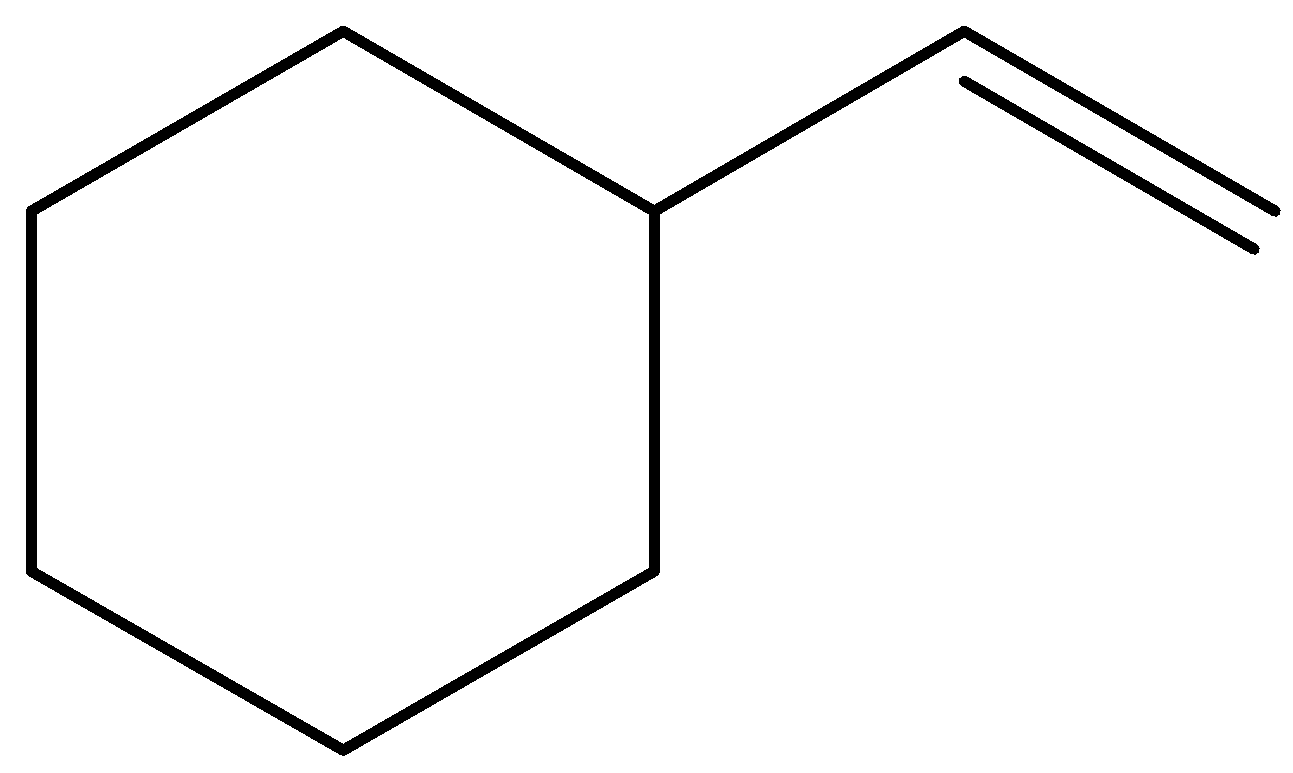
The hydrogenation of molecule (B) would be highly exothermic.
So, the correct answer is Option B.
Note:
We must know that the stabilizing effect because of the bonding interactions between a filled carbon-hydrogen orbital and an empty neighbouring orbital is known as hyperconjugation. Increase in the number of hyperconjugation interactions is seen, when the substitution of an alkene increases.
