Question
Question: In Victor Meyer’s test, the colour given by \({1^0}\) , \({2^0}\) and \({3^0}\) alcohols are respect...
In Victor Meyer’s test, the colour given by 10 , 20 and 30 alcohols are respectively
A) red, colourless, blue
B) red, blue, colourless
C) colourless, red, blue
D) red, blue, violet
Solution
Victor Maeyer’s test is used to differentiate primary, secondary and tertiary alcohols. In Victor Maeyer’s test, alcohols are treated with the reagents P+I2 , AgNO2, HONO and NaOH in the steps one by one. The colours obtained with the alkali helps us to distinguish the alcohols.
Complete step by step solution:
10 , 20 and 30 alcohols can be distinguished using the Victor Maeyer’s test. Victor Maeyer’s test is done in four steps with the reagents P+I2 , AgNO2, HONO and NaOH respectively.
In Victor Maeyer’s test, the alcohols are treated in the following steps.
Step 1: Firstly, the sample alcohol is treated with the reagent P+I2 to get the iodoalkane as a product.
Step 2: The iodoalkane obtained is then treated with AgNO2 solution to get the nitroalkane.
Step 3: The nitroalkane obtained is then treated with HONO (nitrous acid).
Step 4: The resulting solution in step 3 is then treated with the alkali like NaOH and the colour is obtained.
Let us now do Victor Maeyer’s test of primary, secondary and tertiary alcohols.
- Victor Maeyer’s test of 1o alcohols:
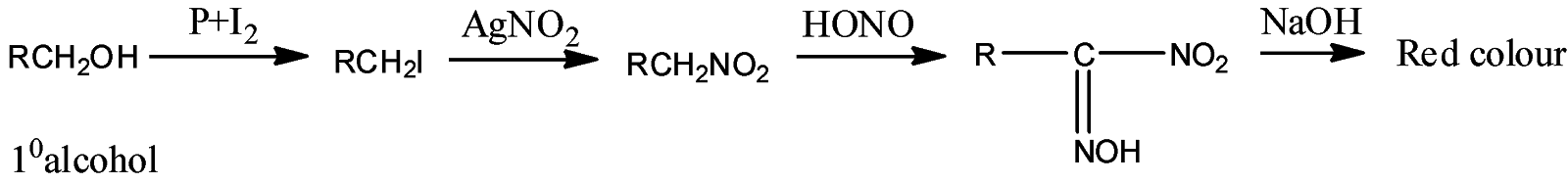
- Victor Maeyer’s test for 2o alcohols:
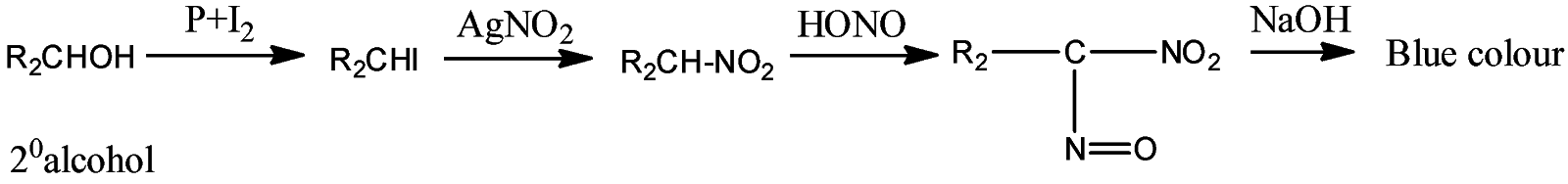
- Victor Maeyer’s test for 3o alcohols:
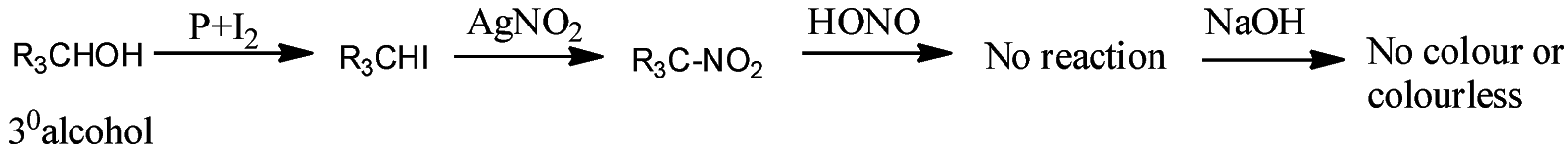
Thus, we can conclude from the above reactions of Victor Maeyer’s test of 10, 20 and 30 alcohols that, primary alcohols (1o) gives the red colour solution with alkali, secondary alcohols (2o) gives the blue colour solution with alkali, and tertiary alcohols (3o) gives the colourless solution.
Thus, option (B) is the correct answer.
Note: Nitrous acid (HONO) is the combination of two reagents NaNO3 and H2SO4. A key point to note is that Victor Maeyer’s test is not given by phenols because the procedure involves the breaking of OH bonds with carbon but in case of phenol, a carbon-oxygen bond is much stronger, having a partial bond character.
