Question
Question: In variable conservative potential energy field in a confined space, an electron has total mechanica...
In variable conservative potential energy field in a confined space, an electron has total mechanical energy of E₀. The potential energy is −2E0 for half the space and 2E0 for the remaining half. Find ratio of largest de Broglie wavelength to smallest one for electron in given space
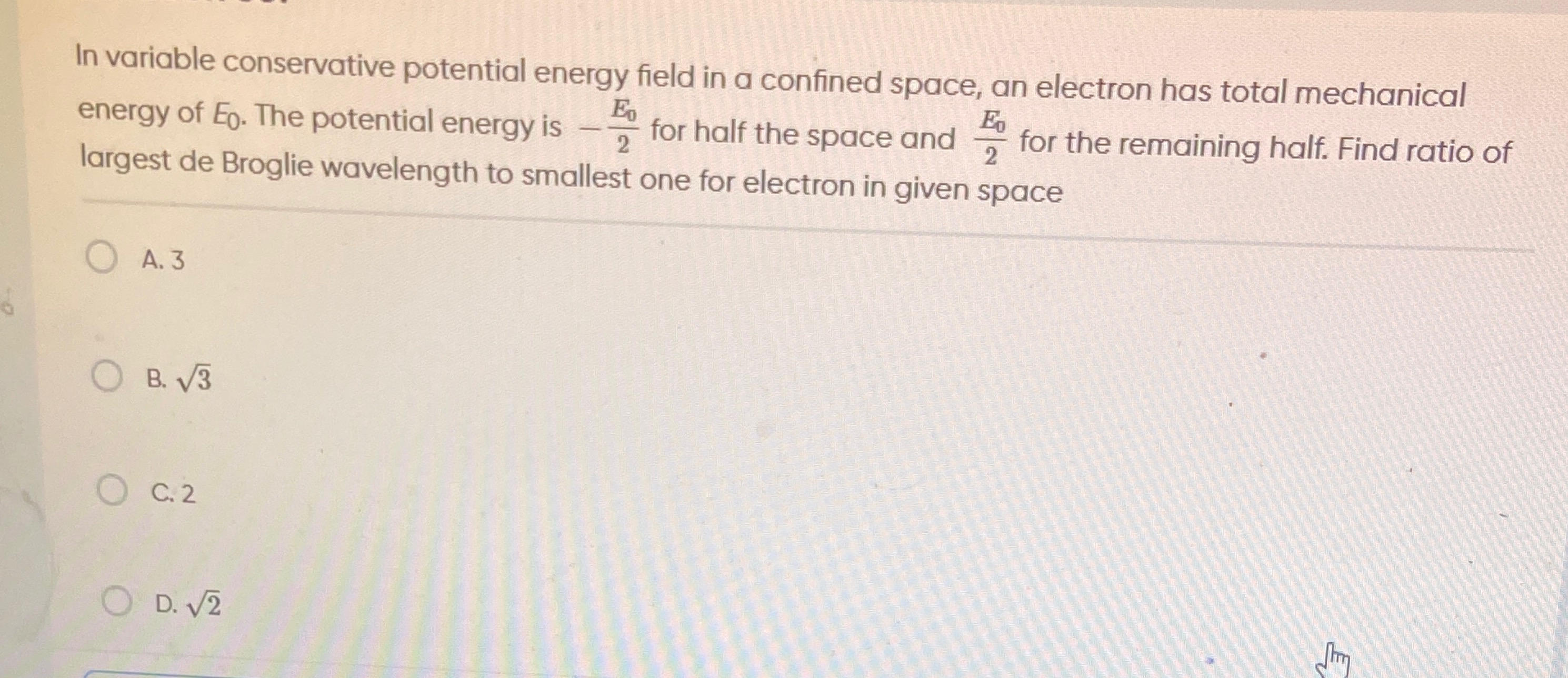
3
√3
2
√2
3
Solution
The de Broglie wavelength λ is related to the momentum p of a particle by λ=ph. The kinetic energy K is related to momentum by K=2mp2, which implies p=2mK. Substituting this into the de Broglie wavelength formula gives λ=2mKh. This shows that the de Broglie wavelength is inversely proportional to the square root of the kinetic energy (λ∝K1). Therefore, the largest wavelength corresponds to the smallest kinetic energy, and the smallest wavelength corresponds to the largest kinetic energy.
The total mechanical energy E of the electron is given as E0. The kinetic energy K in any region is given by K=E0−U, where U is the potential energy in that region. For the electron to exist in a region, its kinetic energy must be non-negative (K≥0), which implies E0≥U.
Region 1: Potential Energy, U1=−2E0. Kinetic Energy, K1=E0−U1=E0−(−2E0)=E0+2E0=23E0. For K1≥0, we must have E0≥0.
Region 2: Potential Energy, U2=2E0. Kinetic Energy, K2=E0−U2=E0−2E0=2E0. For K2≥0, we must have E0≥0.
Assuming E0>0 (as E0=0 would lead to infinite wavelengths, and negative E0 would lead to negative kinetic energy), we compare the kinetic energies: K1=23E0 K2=2E0
Clearly, K2<K1. Thus, the smallest kinetic energy is Kmin=K2=2E0, and the largest kinetic energy is Kmax=K1=23E0.
The largest de Broglie wavelength (λlargest) will occur where kinetic energy is smallest (K2), and the smallest de Broglie wavelength (λsmallest) will occur where kinetic energy is largest (K1). So, λlargest∝K21 and λsmallest∝K11.
The ratio of the largest de Broglie wavelength to the smallest one is: λsmallestλlargest=1/K11/K2=K2K1 Substituting the values of K1 and K2: λsmallestλlargest=E0/23E0/2=13=3
The ratio of the largest de Broglie wavelength to the smallest one is 3.
