Question
Question: In urotropine, the number of \(-N-N-\) bonds is: (a)- 6 (b)- 4 (c)- 2 (d)- 0...
In urotropine, the number of −N−N− bonds is:
(a)- 6
(b)- 4
(c)- 2
(d)- 0
Solution
Urotropine is also known as hexamethylenetetramine. The molecular formula of urotropine is(CH2)6N4. It has a cage-like structure with 6 N−CH2−N bonds.
Complete answer:
The urotropine or hexamine is also known as Hexamethylenetetramine or methenamine. It is a heterocyclic organic compound. It has a formula of(CH2)6N4.
Urotropine is a white crystalline compound. It is highly soluble in water and polar organic solvents.
It has a cage-like structure. It is used in the synthesis of chemical compounds like rubber additives, plastics, pharmaceuticals, etc.
It is prepared by reacting formaldehyde with ammonia. This reaction can be conducted in the gas phase as well as in the solution.
The reaction is given below:
4NH3+6HCHO→C6H12N4+6H2O
It has a symmetric tetrahedral cage-like structure.
The structure is given below:
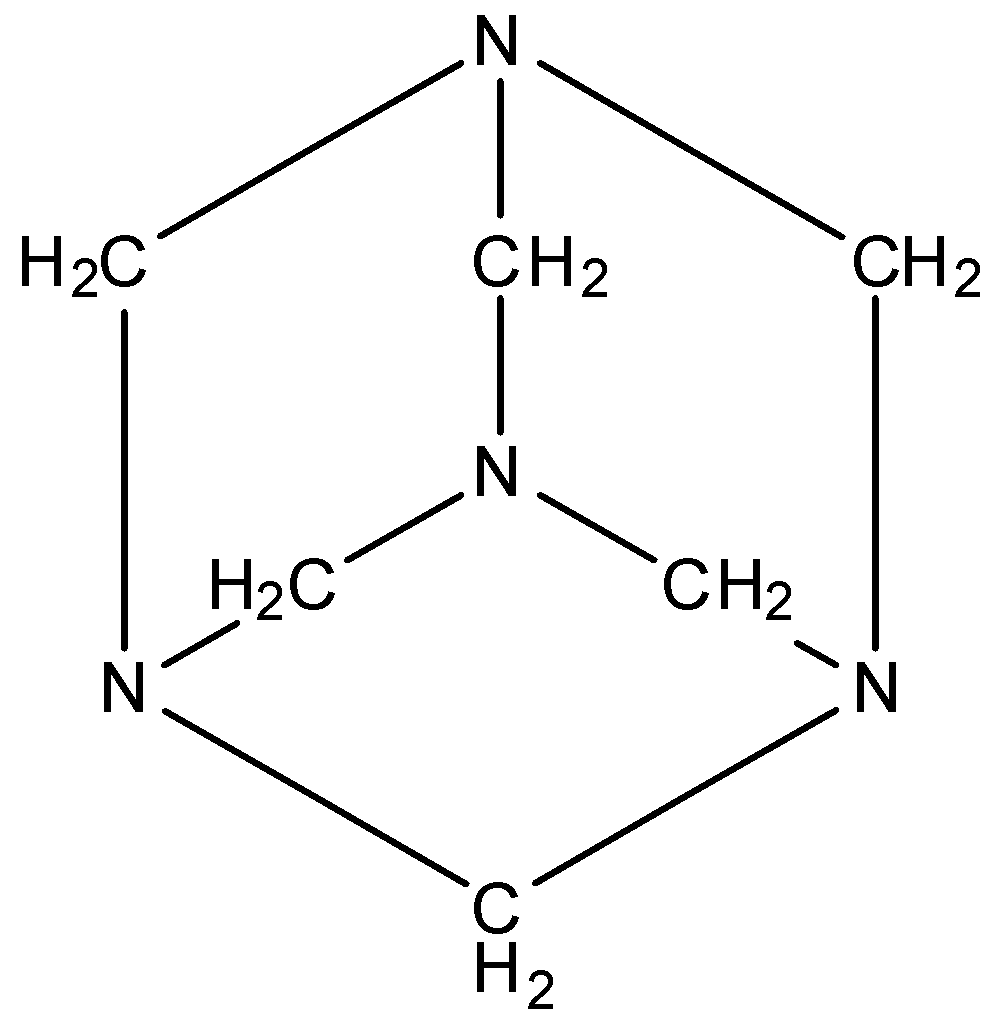
It doesn’t have any space or void in between for other molecules to bind.
It undergoes protonation and behaves like an amine base.
So, in the structure, you can see that there are 6 bonds and each bond is N−CH2−N.
The urotropine is used for medical purposes as mandelic acid salt used for the treatment of urinary tract infection. It is also used as a preservative for food.
So, the molecule of urotropine doesn't have a −N−N− bond.
Hence, the correct answer is an option (d)- 0.
Note:
Urotropine is a versatile reagent used in much organic synthesis. It is used in reactions like the Duff reaction (formylation of arenes), in Sommelet reaction (converting benzyl halides to aldehydes), and in Delepine reaction (synthesis of amines from alkyl halides).
