Question
Question: In this reaction\(Me - C \equiv C - Et\xrightarrow{{Na/liq.N{H_3}}}P\xrightarrow[{CC{l_4}}]{{B{r_2}}...
In this reactionMe−C≡C−EtNa/liq.NH3PBr2CCl4Q; then Q is:
A. a pure compound which is optically inactive due to internal compensation
B. a binary mixture which is optically inactive due to external compensation
C. a binary mixture which is optically active
D. a pure compound which is optically inactive due to absence of chiral center
Solution
To solve this question, first we need to understand the meaning of optically inactive. A racemic mixture is optically inactive. A racemic mixture is one which contains two enantiomers in d and l-forms in equal proportions. Thus, the rotation due to one isomer gets cancelled by the rotation due to another. Therefore, it has zero optical rotation. So, the mixture is called optically inactive.
Complete step by step answer:
To find the value of Q, we will solve the first part of the equation given in the question.
Me−C≡C−EtNa/liq.NH3P
When Na or liq. NH3 is added then trans-reduction takes place. When we add sodium or ammonia to the reaction, it is known as dissolving metal reduction reaction. In this reaction, the electrons from Na metal sequentially add to the alkyne that further results in an anion that is protonated by the NH3 solvent.
So, the compound formed in the first part i.e. P is,
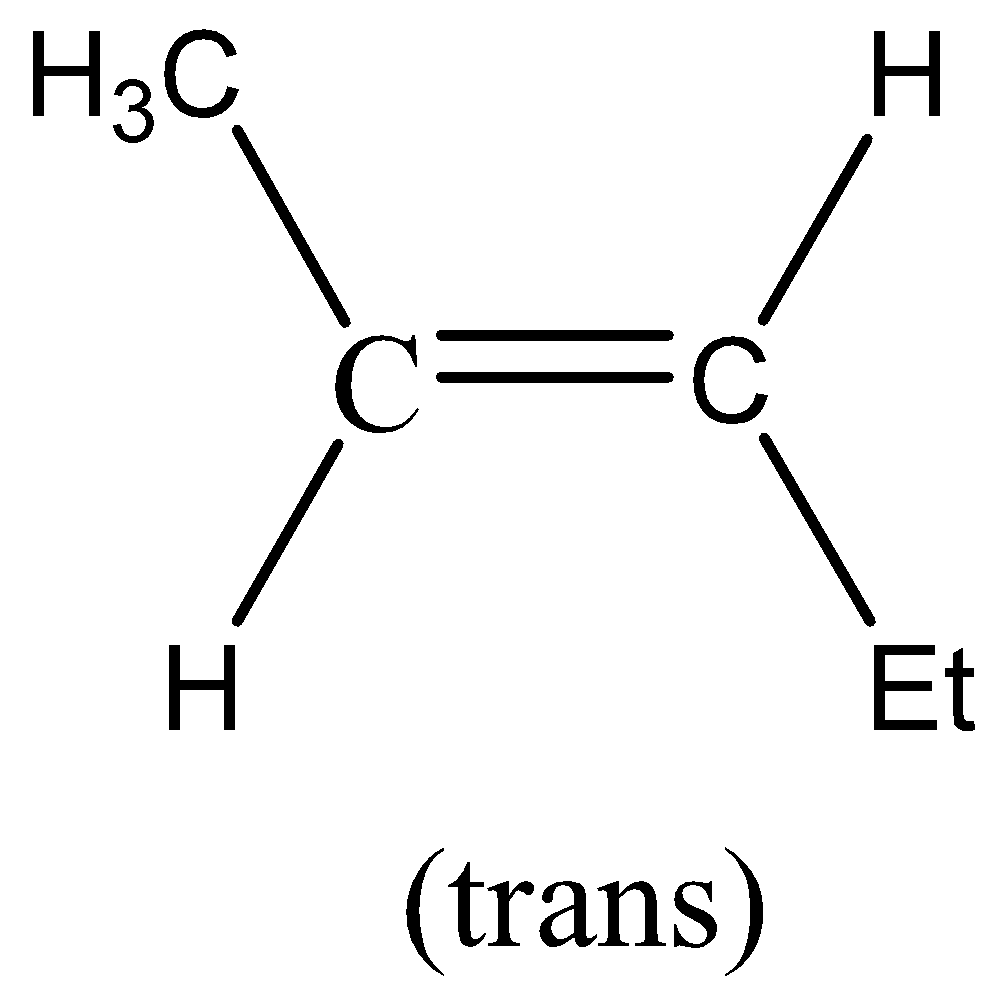
Now, with the help of this we will find the other find of the reaction i.e. Q.
PBr2CCl4Q
Here, we can see 2 Br atoms are taken so the compound P is divided into two different parts. Then, anti-reduction takes place due to which mirror images are formed in the compound Q. Now, we rotate the first and second part of compound Q move in clockwise and anticlockwise direction. Due to this movement, these compounds are optically inactive due to external compensation.
So, the racemic mixture in this reaction Q is:
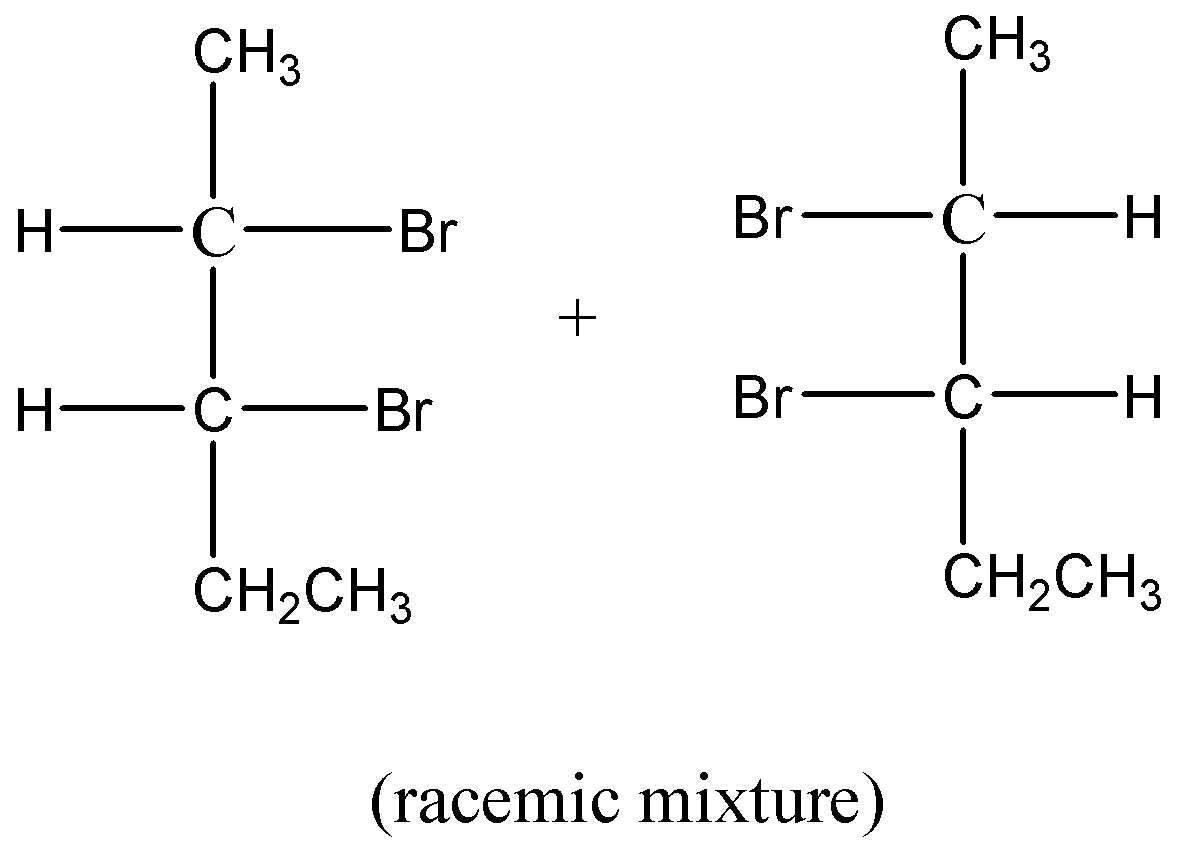
Therefore, we can say that the binary mixture which is optically inactive due to external compensation. Hence option B is correct.
Note: After solving, this question we need to keep in mind, when Br2 and CCl4 are involved in this reaction then an induced dipole interaction between the molecules and bromine gives Br+ and Br−, Br+ attacks as an electrophile on alkene then undergoes electrophilic addition reaction.
