Question
Question: In the structure of \[I{{F}_{7}}\] (Iodine heptafluoride) ----------. (A) \[{{d}_{xy}},{{d}_{x}},{...
In the structure of IF7 (Iodine heptafluoride) ----------.
(A) dxy,dx,dz orbitals are involved in hybridization
(B) Axial bonds are longer than equatorial bonds
(C) There are 10 different orthogonal angels
(D)The resultant geometry is a decahedral
Solution
Iodine heptafluoride, also called iodine (VII) fluoride. It is an interhalogen compound having a chemical formula ofIF7. As per VSEPR theory the structure of IF7 is pentagonal bipyramidal with a hybridization of sp3d3.
Complete step by step solution:
The structure of IF7is as follows.
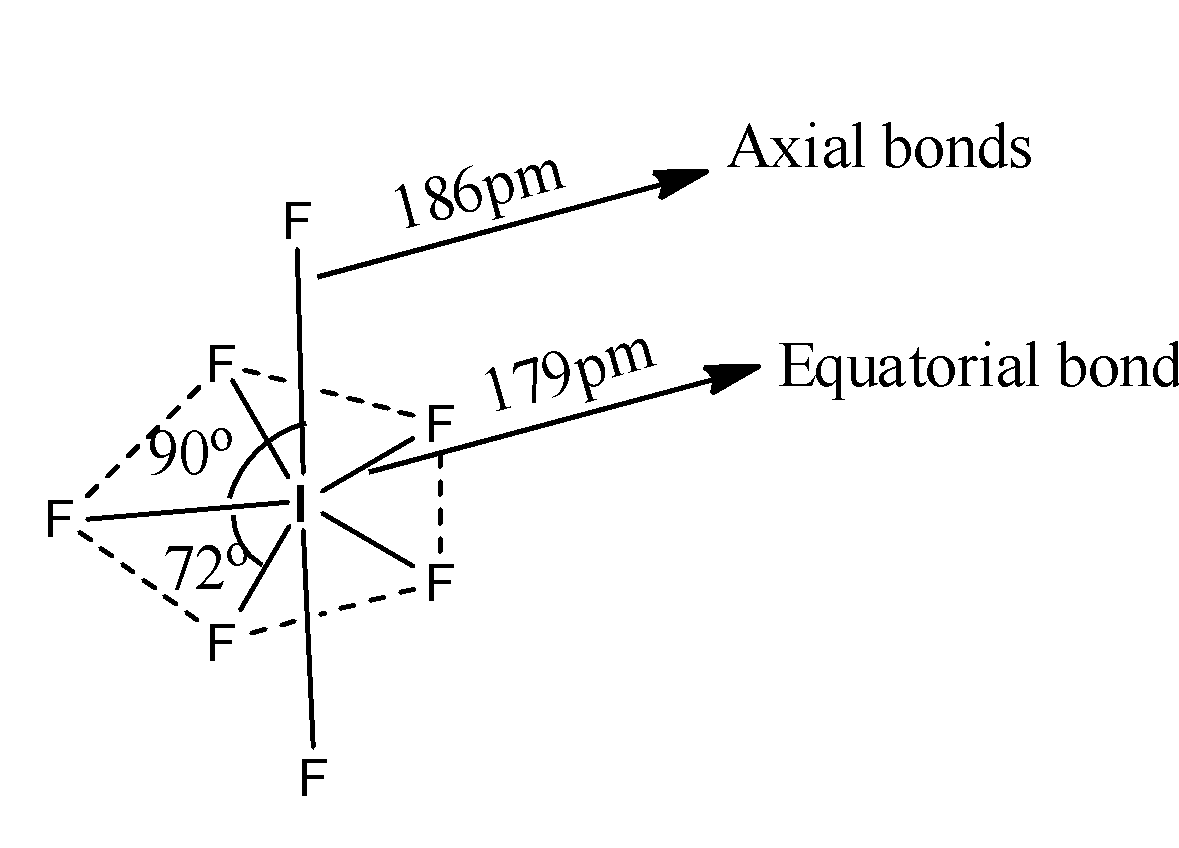
The hybridization of Iodine in IF7 is sp3d3 and the overlapping of fluorine with iodine orbitals we can see as follows. 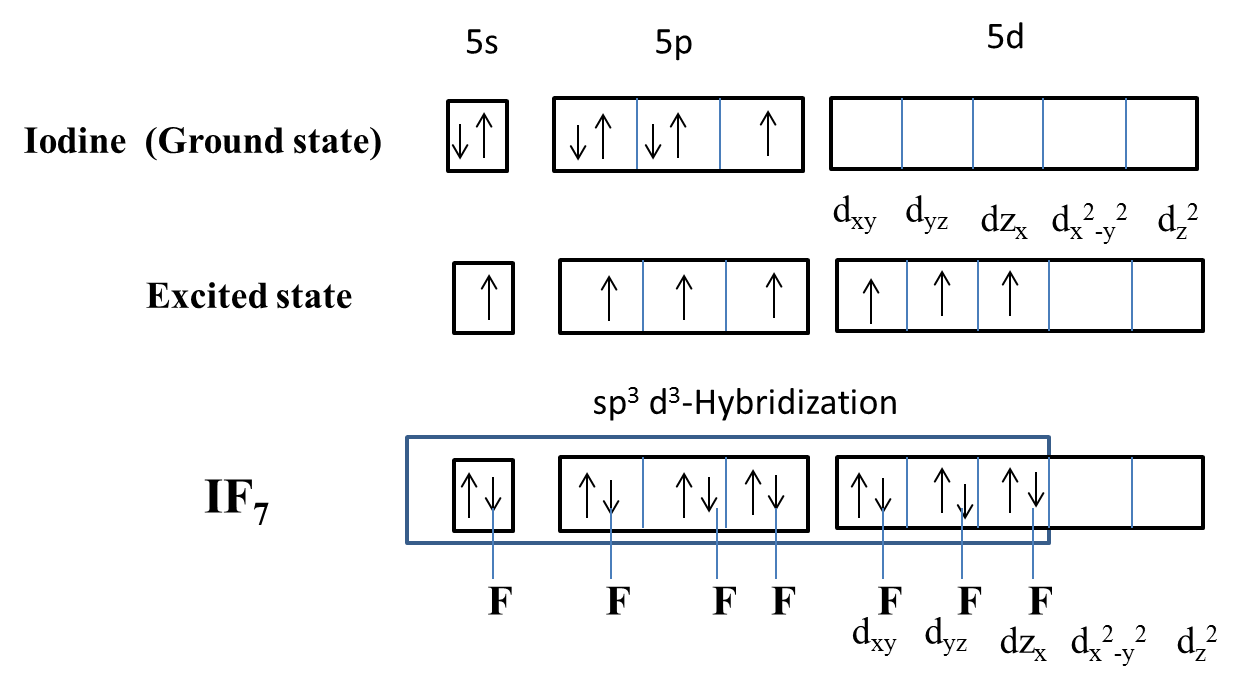
Now coming to the given options, option A, dxy,dx,dz orbitals are involved in hybridization, it is wrong because in the hybridization the orbitals involved are dxy,dyz,dzx not dxy,dx,dz. So, option A is wrong.
Coming to option B, Axial bonds are longer than equatorial bonds, yes it is true. Because from the structure we can say that the length of the axial bonds are 186pm, and length of the equatorial bonds are 179pm. So, axial bonds are longer than equatorial bonds. So, option B is correct.
Coming to option C, There are 10 different orthogonal angels. No there are only two types of orthogonal angles, there are 90 and 72. So, option C is wrong.
Coming to option D, The resultant geometry is decahedral, it is wrong. Because the resultant geometry is pentagonal bipyramidal. So, option D is wrong.
So, the correct option is B.
Note: The structure of theIF5is a square pyramid or distorted octahedral and the structure of theIF7 is pentagonal bipyramidal. Both structures are different.
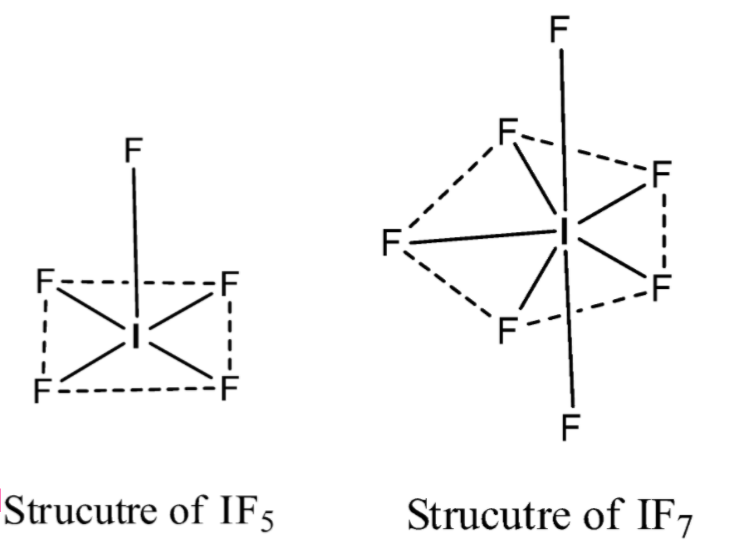
The hybridization of Iodine in IF5issp3d2
The hybridization of Iodine in IF7issp3d3
