Question
Chemistry Question on Chemical bonding and molecular structure
In the structure of ClF3, the number of lone pairs of electrons on the central atom Cl is:
A
three
B
one
C
four
D
two
Answer
two
Explanation
Solution
The correct answer is Option D) two
The structure of ClF3 is
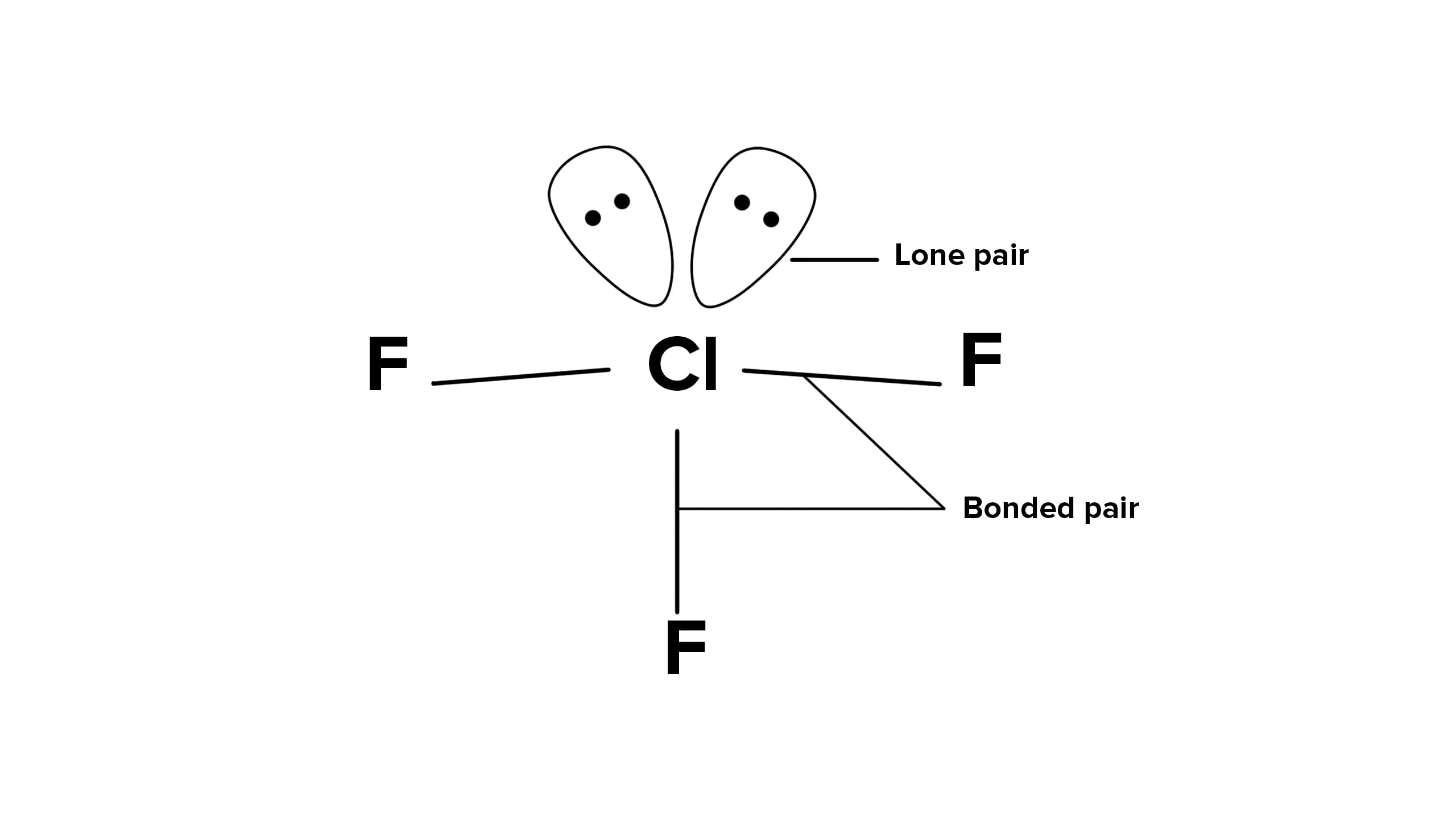
In the structure of ClF3, the number of lone pairs of electrons on the central atom Cl is two. Cl has 7 valence electrons out of which 3 are involved in bond formation with 3 F atoms. 7−3=4 valence electrons remain in the form of 2 lone pairs of electrons.
