Question
Question: In the structure of borax, the numbers of boron atoms and \(B - O - B\) units, respectively, are: ...
In the structure of borax, the numbers of boron atoms and B−O−B units, respectively, are:
(A) 4and5
(B) 4and3
(C) 5and4
(D) 5and3
Solution
To solve this question, we must first understand the structure of Borax. Then we need to assess its whole structure and look for the required queries and then only we can conclude the correct answer.
Complete step-by-step answer: Before we move forward with the solution of this given question, let us first understand some basic concepts:
Borax: is a compound consisting of an elementary substance called boron, united to oxygen and soda. Its formula is also known as sodium borate formula, sodium tetraborate formula or disodium tetraborate formula is explained in this article. Borax is a soft, colourless compound of Boron and can be dissolved in water. The main forms of borax are anhydrous and decahydrate salt and sometimes as pentahydrate salt.
Borax is naturally found in evaporite deposits which are produced by the recurrent evaporation of seasonal lakes. Naturally occurring borax is purified by recrystallization. Borax can also be synthesized from boron compounds.
Structure of Borax:
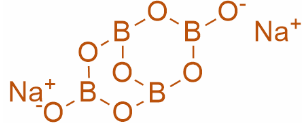
Clearly, from the structure we can observe that there are 4 Boron atoms and 5 B−O−B units.
So, clearly we can conclude that the correct answer is Option (A).
Note: Borax is a component of many detergents, cosmetics, and enamel glazes. It is used to make buffer solutions in biochemistry, as a fire retardant, as an anti-fungal compound, in the manufacture of fiberglass, as a flux in metallurgy, neutron-capture shields for radioactive sources, a texturing agent in cooking, as a cross-linking agent in slime, as an alkali in photographic developers, as a precursor for other boron compounds, and is useful as an insecticide.
