Question
Question: In the spinel structure, oxides ions are cubically-closed packed whereas \({{\dfrac{1}{8}}^{th}}\)of...
In the spinel structure, oxides ions are cubically-closed packed whereas 81thof tetrahedral voids are occupied by A2+cation and half of octahedral voids are occupied by B3+cations. The general formula of compound having spinel structure is:
(1) A2B2O4
(2) AB2O4
(3) A2B4O2
(4) A4B2O2
Solution
Generally, tetrahedral voids are two times octahedral voids in cubic close packed structures.
Complete step by step solution:
Let us first understand about the packed structures and voids present within them.
Cubic close packed structures-
The cubic close packed structure consists of four layers of atoms per unit cell. There are two types of arrangement of atoms in the structure i.e. Simple cubic arrangement and Hexagonal cubic arrangement.
Simple cubic arrangement has the same layer of atoms arranged layer by layer one above the other in a definite manner.
Hexagonal cubic arrangement has one layer in the depression of another layer, repeating over one another.
There are two types of gaps (commonly known as voids) within them;
Tetrahedral void-
These are mostly found in CCP and are triangular in shape with coordination number of 4.
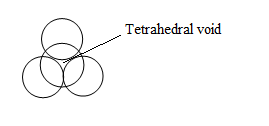
Tetrahedral voids are two times the number of atoms in the unit cell.
Octahedral void-
These are mostly found in HCP and are octahedral in shape with coordination number of 6.
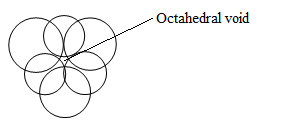
Octahedral voids are equal to the number of atoms in the unit cell.
Thus,
Tetrahedral voids are two times the octahedral voids.
Spinel group-
These are the group of minerals with general formulation AB2X4.
where,
X is anion
A and B are cations.
Illustration,
Oxides form close packings. Thus, it is an anion.
Whereas, A2+ and B3+ are cations.
Given that,
81thof tetrahedral voids are occupied by A2+cation;
Thus, A2+ ions will be 41thof oxide ions.
And half of octahedral voids are occupied by B3+cations;
Thus, B3+ ions will be 21 of oxide ions.
Hence, A:B:O = 41: 21: 1 = 1:2:4.
Therefore, the formula is AB2O4.
Option (B) is correct.
Note: In accordance with the hint given, we can ignore option (A) and (D) from the start. Only concentrate on the remaining two options.
