Question
Question: In the sigmatropic rearrangement shown below, which of the following choices explains the difference...
In the sigmatropic rearrangement shown below, which of the following choices explains the difference in products for thermal and photochemical conditions?
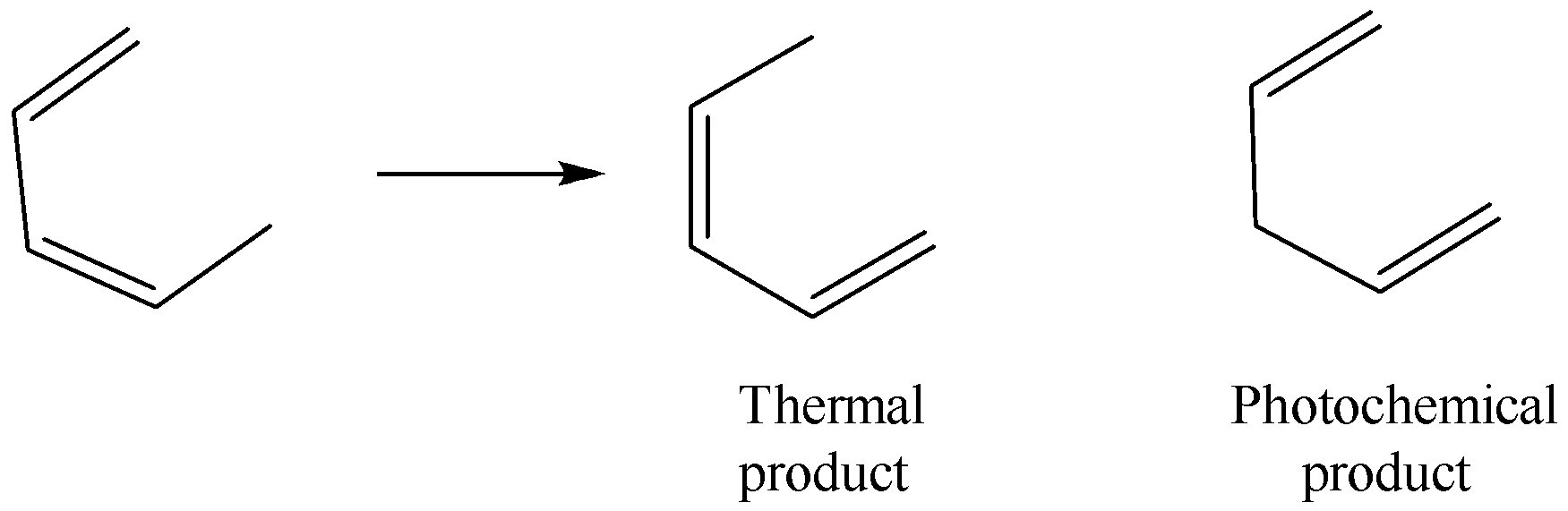
A. The photochemical product is too strained to form unless an electron in the starting material is promoted to a higher energy orbital.
B. The photochemical product only forms when irradiated with light because the product is higher energy than the starting material.
C. The photochemical product only forms when irradiated with light because the thermal product is identical to its starting material.
D. Orbital symmetry arguments allow for the 1,3-rearrangement to occur only from the photochemical HOMO and a 1,5- rearrangement to occur only from the original (thermal) HOMO
Solution
Photochemical reactions proceed in a different manner than the temperature-driven reactions or the thermal reactions. In paths of photochemical reactions, higher energy intermediates are formed thermally. These types of reactions are found in everyday life like in the process of photosynthesis, and formation of vitamin D in humans by exposure to the sunlight.
Complete step by step answer:
The photochemical product is too strained to form.
A photochemical reaction is a type of chemical reaction started or triggered when light energy is absorbed by a substance’s molecules.
This response leads the molecules to experience a temporary excited state, thus altering their properties from the substance’s initial molecule.
Photochemical products are higher than the starting material.
The photochemical reaction initiates with the absorption of light where light is consisting of photons. When the molecules of the reactant species absorb energy in this way, it causes the molecule to get excited to a high energy state in which the chemical and physical properties of the molecule are different from that of the original molecule.
These new chemical species may change to a new structure, it can combine with each other and can transfer their electronic excitation energy to other molecules. In Excited states, they are stronger acids and strong reductants than the original ground state.
Photochemical product and thermal product.
The reactions that take place by the absorption of light energy are called photochemical reactions. Generally, it takes place by the absorption of ultraviolet light, visible light, or infrared radiation.
In thermal reaction is a form of chemical reaction in which the reactants get energy as heat. It mainly decomposes a substance when we apply heat energy.
Orbital symmetry arguments.
In a thermal reaction, the reactant is in its ground state (HOMO) whereas the reactants are in excited state (LUMO) in a photochemical reaction.
The ground-state that is, HOMO and the excited-state LUMO always have the exact opposite symmetries- if one is symmetric and the other is asymmetric.
Pericyclic reactions involve the migration of a sigma bond from one site to another within the same molecule. Such types of reactions are called sigmatropic rearrangements.
So, the correct answer is Option B,C.
Note: Sigmatropic rearrangements is a type of reaction in which a sigma bond that is flanked by one or more pi bonds gets migrated to a new position. The product will produce higher energy products due to the absorption of light.
