Question
Question: In the Rosenmund’s reaction, \(RCOCl+{{H}_{2}}\xrightarrow{Pd/BaS{{O}_{4}}}RCHO+HCl\) Here \(Ba...
In the Rosenmund’s reaction,
RCOCl+H2Pd/BaSO4RCHO+HCl
Here BaSO4 :
(a)- promotes the catalytic activity of Pd
(b)- removes the HCl formed in the reaction
(c)- deactivates palladium
(d)- activates palladium
Solution
Acid chlorides are converted into aldehydes in the presence of palladium and barium sulfate. Palladium is a strong reducing agent. It reduces the aldehydes to further alcohol. So, it has to be stopped at the stage of the aldehydes.
Complete answer:
Acid chlorides are easily reduced to the corresponding aldehydes by passing hydrogen gas through boiling xylene solution of the acid chloride in presence of Pd catalyst supported over BaSO4 and partially poisoned by the addition of sulfur and quinoline.
The reactions are given below:
Acid chlorides are converted into aldehydes.
RCOCl+H2Pd,BaSO4,SBoiling xyleneRCHO+HCl
Example: Acetyl chloride is converted into acetaldehyde.
CH3COCl+H2Pd,BaSO4,SBoiling xyleneCH3CHO+HCl
Benzoyl chloride is converted into benzaldehyde.
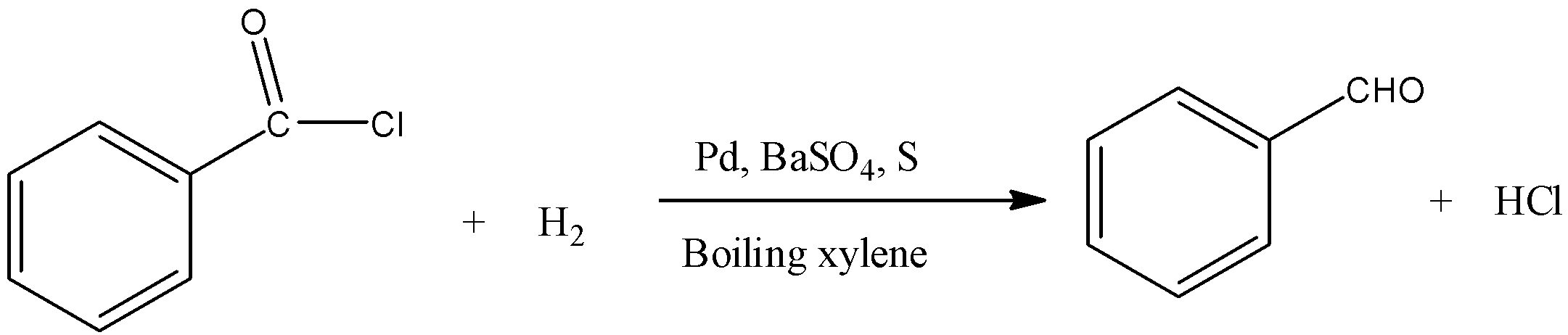
Normally, aldehydes are further reduced to primary alcohols. But the addition of BaSO4 and sulfur (or quinoline) poisons the palladium catalyst and thus does not permit the further reduction of aldehydes to alcohols.
Hence the correct answer is an option (c)- deactivates palladium.
Additional information:
Acid chlorides can be converted into aldehydes by lithium-tert-butoxyaluminum hydride [LiAlH(O−t−Bu)3] at 196K.
The reaction is given below:
RCOCl\xrightarrow[{{H}_{3}}{{O}^{+}}]{LiAlH{{(O-t-Bu)}_{3}},dry\text{ }ether,196K}RCHO
It may be noted that [LiAlH(O−t−Bu)3]is less reactive than LiAlH4 because the electron-withdrawing t-butoxy group stabilizes the negatively charged aluminium ion. Therefore, it reduces the more reactive acid chlorides to aldehydes but does not reduce the less reactive aldehydes to primary alcohols.
Note: With the rosenmund reaction formaldehyde cannot be prepared since formyl chloride, HCOCl is unstable to room temperature. This reaction is used only for the preparation of aldehydes but not for ketones.
