Question
Question: In the reduction of nitriles to prepare primary amine. A. \(LiAl{H_4}\) is used. B. \(NaB{H_4}\)...
In the reduction of nitriles to prepare primary amine.
A. LiAlH4 is used.
B. NaBH4 is used
C. Sn/HCl is used
D. None of these.
Solution
We all know that the lithium aluminium hydride is one of the most important and useful reagents for the reduction of carbonyl compounds and nitriles. We have to remember that the nitriles are the organic compounds which also are called Cyano Compounds. These classes of organic compounds contain the functional group (−C ≡ N) and it's attached to the atom. Some nitriles are mainly prepared by heating carboxylic acids with the ammonia compound in presence of some catalysts.
Complete answer:
We must remember that the nitrile reacts with the lithium tetrahydridoaluminate in solution in ethoxyethane followed by treatment of the merchandise of that reaction with a dilute acid. As a result the carbon-nitrogen triple bond is reduced to offer a primary amine.
For instance, with ethane nitrile we get ethylamine:
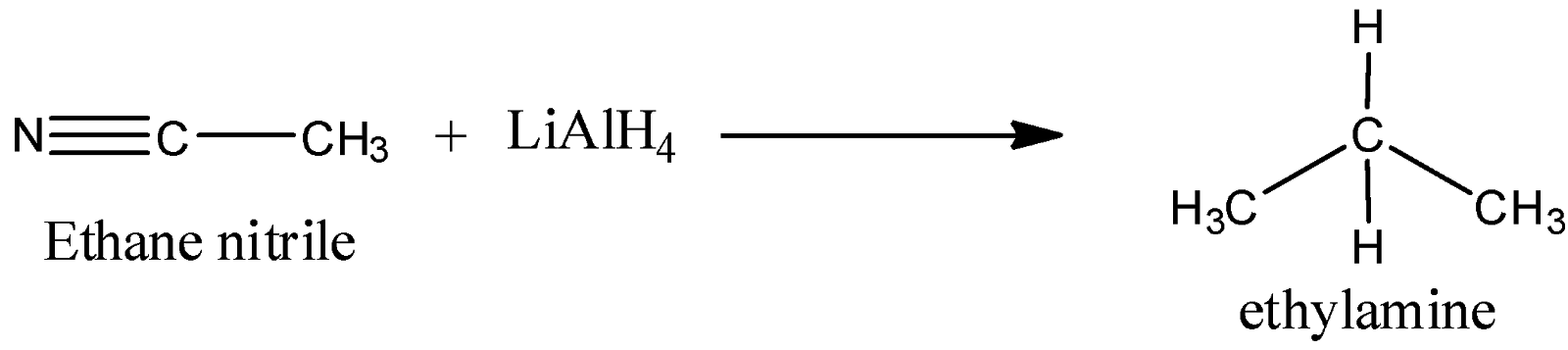
As we know that the sodium borohydride is not a strong adequate reducing agent to reduce nitriles. Hence option B is incorrect.
We must remember that the nitriles are reduced to aldehydes using Sn/HCl as a reducing agent. This is known as the Stephen aldehyde synthesis. Hence option C is incorrect.
So, the correct answer is “Option A”.
Note:
This is often a process which is used within the manufacturing of nitriles from oils and fats, these products are getting used because the softening agents in substances like synthetic rubbers, textiles, plastics and also within the making of amines. Nitriles compounds also are formed by the method of heating amides with the phosphorus pentoxide compound. They will even be reduced to some primary amines by reacting lithium aluminum hydride or getting it hydrolyzed to carboxylic acids in presence of acid or base.
