Question
Question: In the reaction the gas lost during effervescence is/are: 
(A) C13O2
(B) C12O2
(C) H2+C12O2
(D) H2+C13O2
Solution
Hint : We know that the sodium benzoate is an aromatic chemical compound with chemical formula C7H5NaO2/PhNaO2 . It is a colourless and odourless crystalline powder. It is not found naturally so is produced by the neutralization reaction of benzoic acid with sodium hydroxide
Complete Step By Step Answer:
Here due to its antibacterial properties the compound sodium benzoate is used as preservatives and as also in pharmacy. The parent compound of sodium benzoate, i.e. the benzoic acid itself is a good preservative. But when added to the sodium hydroxide it increases its solubility in different solvents. So the salt formed i.e. sodium benzoate works more efficiently as the food preservative.
The mechanism of dissociation works behind the preservation of food items. When added to the food items it gets dissociated into benzoic acid and sodium chloride. The development of bacteria is inhibited by the benzoic acid and sodium chloride as both are toxic for the microbes.
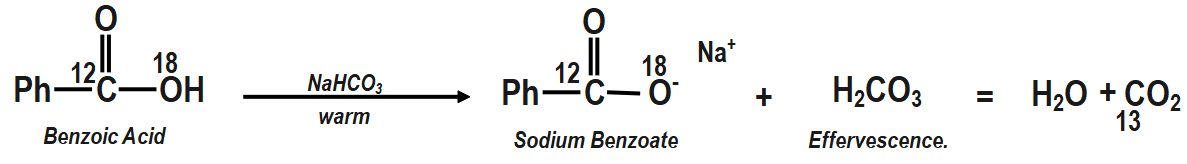
Therefore, correct answer is option A i.e. the gas lost during effervescence is C13O2
Additional Information:
Food preservatives are the chemical compounds which when added to the food items helps to preserve them i.e. make them last longer. The preservatives make the food safe by preventing the growth of mould and bacteria in the food.
Note :
Remember that the sodium benzoate is sodium salt of benzoic acid produced by the neutralization reaction of benzoic acid and sodium hydroxide. It is used as food preservative as it controls the growth of bacteria and mould. Also, it helps to prevent fatty food items from becoming rancid. Although the compound sodium benzoate is not found naturally but benzoic acid is found naturally in various plants and animals.
