Question
Question: In the reaction sequence : $CH_2OH - CHOH - CH_2OH \xrightarrow{KHSO_4/\Delta} (X) \xrightarrow{\fra...
In the reaction sequence : CH2OH−CHOH−CH2OHKHSO4/Δ(X)Δ(C2H5O)3Al(Y).(Y) will be:
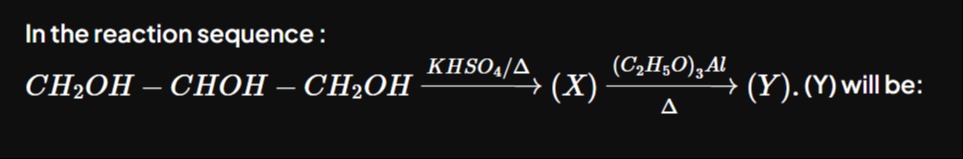
A
Propanal
B
Propanol
C
Allyl alcohol
D
Acrolein
Answer
Allyl alcohol
Explanation
Solution
The reaction sequence involves two steps:
-
Dehydration: Glycerol (CH2OH−CHOH−CH2OH) undergoes dehydration in the presence of KHSO4 and heat (Δ) to form acrolein (CH2=CH−CHO).
CH2OH−CHOH−CH2OHKHSO4/ΔCH2=CH−CHO
-
Reduction: Acrolein is then reduced by aluminum triethoxide ((C2H5O)3Al) to form allyl alcohol (CH2=CH−CH2OH).
CH2=CH−CHOΔ(C2H5O)3AlCH2=CH−CH2OH
Therefore, (Y) is allyl alcohol.
