Question
Question: In the reaction $P_4S_3 \rightarrow P_2O_5 + SO_2$ Equivalent weight of $P_4S_3$ (M = molar mass of ...
In the reaction P4S3→P2O5+SO2 Equivalent weight of P4S3 (M = molar mass of P4S3) is
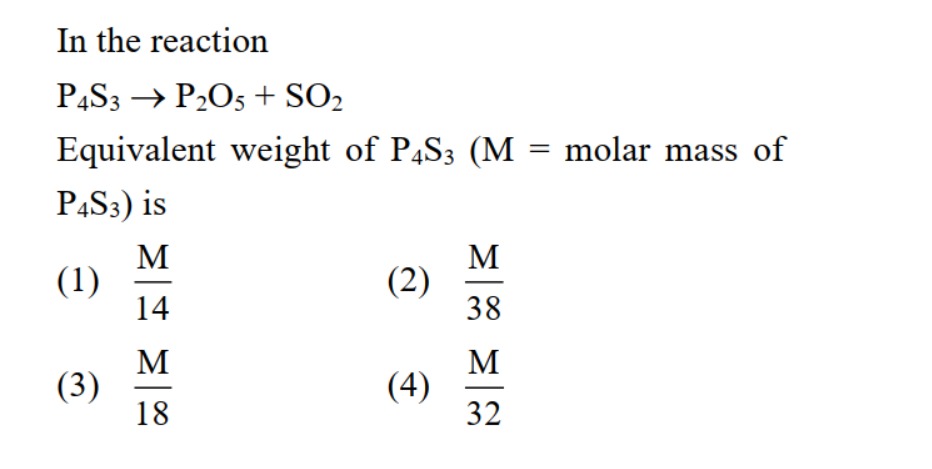
A
M/14
B
M/38
C
M/18
D
M/32
Answer
M/32
Explanation
Solution
The balanced chemical equation for the reaction is: P4S3+8O2→2P2O5+3SO2
To find the equivalent weight of P4S3, we need to determine its n-factor.
-
Oxidation states in P4S3: Assuming the oxidation state of S is -2: 4×OS(P)+3×(−2)=0 4×OS(P)=+6 OS(P)=+1.5 (average)
-
Oxidation states in products: In P2O5: 2×OS(P)+5×(−2)=0⟹OS(P)=+5. In SO2: OS(S)+2×(−2)=0⟹OS(S)=+4.
-
Change in oxidation states:
- Phosphorus (P): Total change for 4 P atoms = 4×(+5)−4×(+1.5)=20−6=+14.
- Sulfur (S): Total change for 3 S atoms = 3×(+4)−3×(−2)=12−(−6)=+18.
-
n-factor: The total change in oxidation states for P4S3 is the sum of the changes for P and S. n-factor = ∣+14∣+∣+18∣=14+18=32.
-
Equivalent Weight: Equivalent Weight = n-factorMolar Mass=32M.
