Question
Question: In the reaction: 
Which of the following compounds will be formed?
A.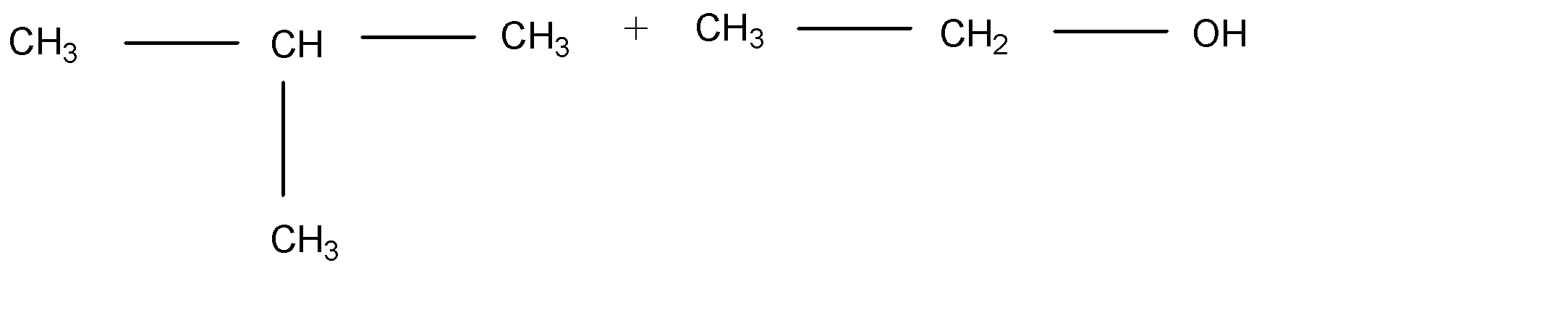
B.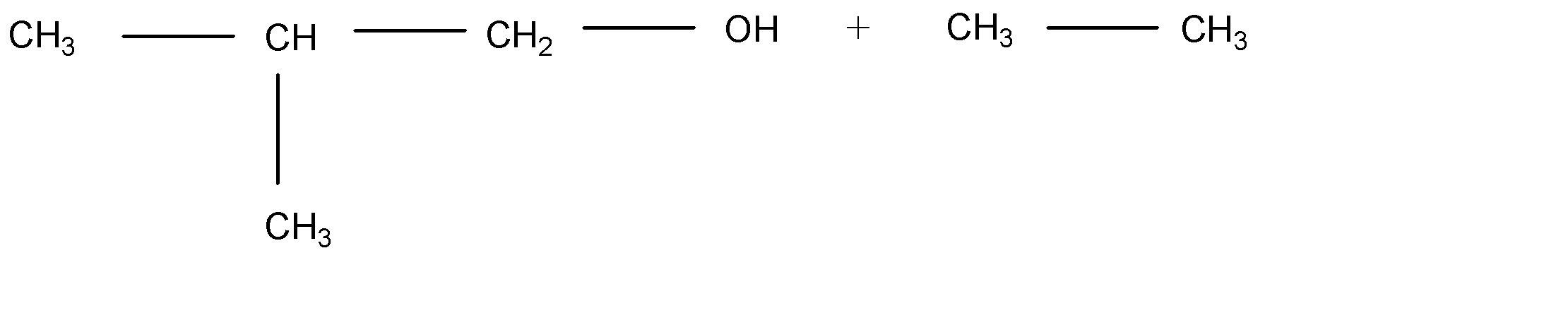
C.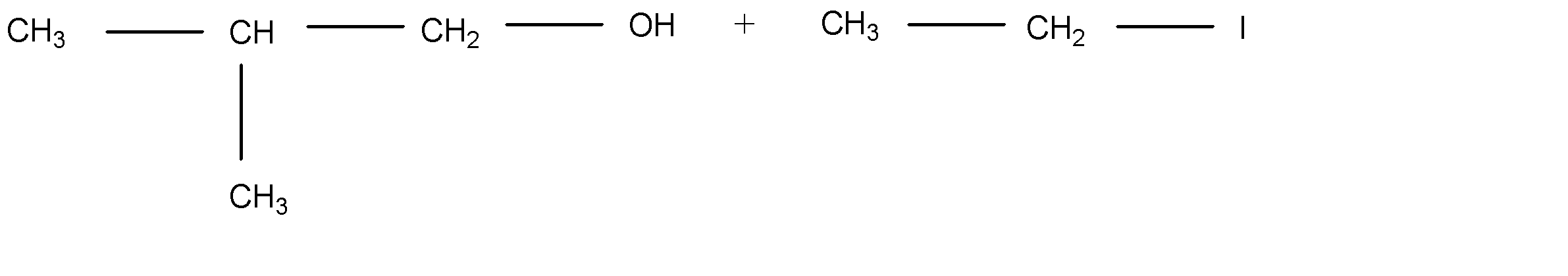
D.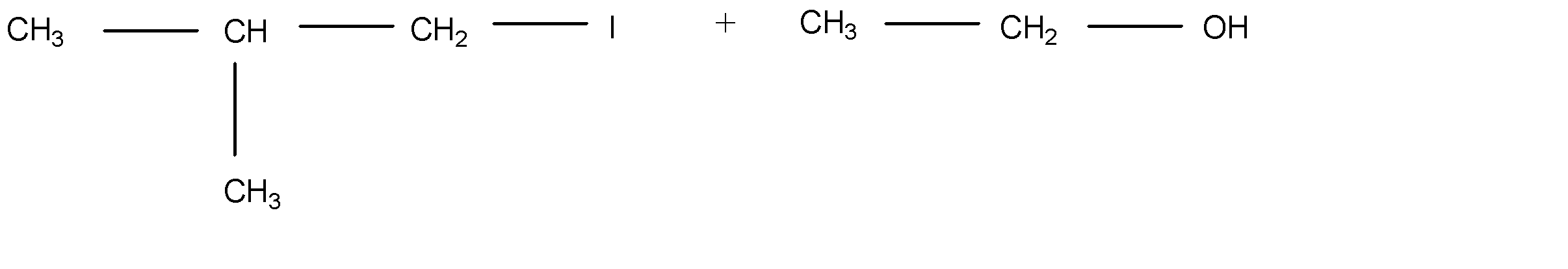
Solution
When hydroiodic gas reacts with ether, the most significant reaction occurs ethers experience. This reaction is a nucleophilic substitution mechanism, so that the reaction of ether with hydroiodic acid gives the product of ethyl alcohol and iodide. There are a number of points in this reaction in different sections that require additional explanations.
Complete step by step answer:
In this reaction, nucleophilic substitution takes place and nucleophilic attack on hydronium ion. Here the SN2 reaction happens and forms the product.
As usual we see the reaction which take place in this given below:
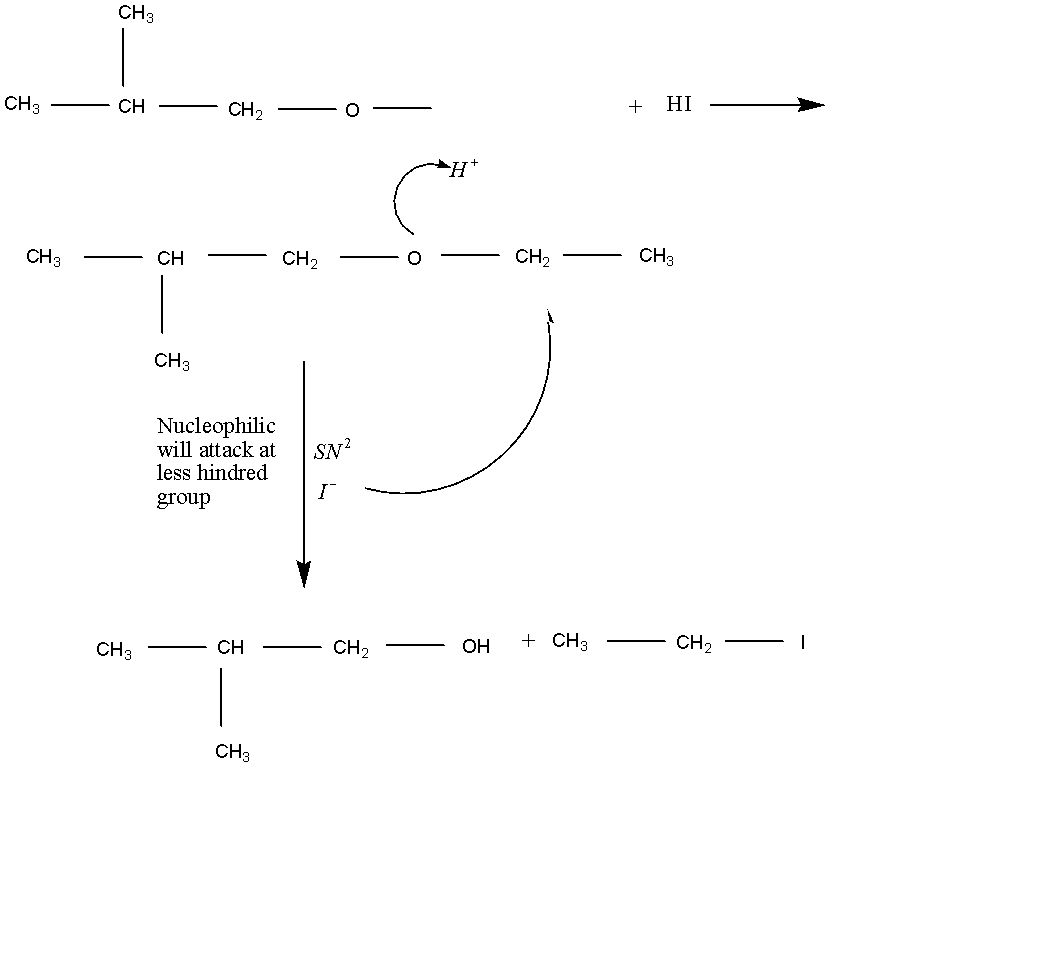
From the above reaction we see that the nucleophile will be attacked at a less hindered group and the SN2 reaction takes place as it forms as the products are alcohol and iodide. As well as we that the most important role in this reaction is nucleophilic agent.
So, Option (C) is the correct answer.
Additional information:
A nucleophile donates electrons in a pair which will be exposed to different kinds of nucleophiles and negatively charged. In SN2 reaction, nucleophile addition and the leaving group occur in taking place in a single step. Nucleophile approaches the carbon atom which the leaving group is attached to.
Note: Nucleophile involves rate determining step of SN2 reaction which reacts faster. Here we must remember that nucleophiles attack in the reactant and form the product. Here nucleophiles increase nucleophilicity. In this reaction we see that interconversion of functional groups. Here second order kinetics followed by SN2 reactions. The group in this reaction which displaced from the carbon known as leaving group and where the substitution takes place in the molecule known as subtracts.
