Question
Question: In the reaction given above, the intermediate is/are: 
(A) 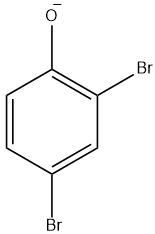
(B) 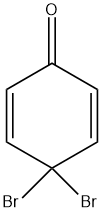
(C) 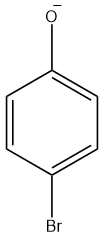
(D) 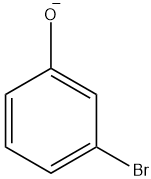
Solution
Electron donating groups are generally ortho/para directors for electrophilic aromatic substitutions, while electron withdrawing groups are generally meta directors with the exception of the halogens which are also ortho/para directors as they have lone pairs of electrons that are shared with the aromatic ring.
Complete step by step answer:
An electron donating group (EDG) or electron releasing group (ERG, Z in structural formulas) is an atom or functional group that donates some of its electron density into a conjugated π system via resonance (mesomerism) or inductive effects (or induction)—called +M or +I effects, respectively—thus making the π system more nucleophilic. As a result of these electronic effects, an aromatic ring to which such a group is attached is more likely to participate in electrophilic substitution reaction. EDGs are therefore often known as activating groups, though steric effects can interfere with the reaction.
Phenol is an ortho/para director, but in a presence of base, the reaction is more rapid. It is due to the higher reactivity of phenolate anion. The negative oxygen was 'forced' to give electron density to the carbons (because it has a negative charge, it has an extra +I effect). Even when cold and with neutral (and relatively weak) electrophiles, the reaction still occurs rapidly.
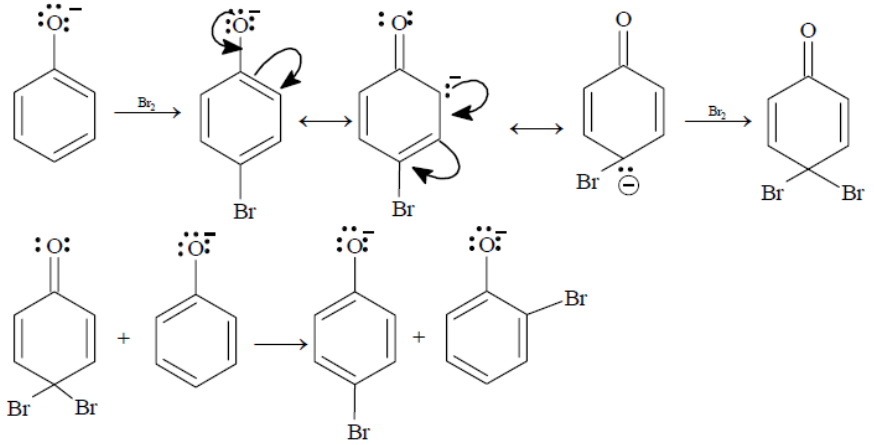
Since, the hydroxyl group is an o, p- directing group, therefore except option D which is a meta derived phenol halide all are the intermediates for the given reaction.
So, the correct answer is “Option A, B and C”.
Note: An electron withdrawing group (EWG) will have the opposite effect on the nucleophilicity of the ring. The EWG removes electron density from a π system, making it less reactive in this type of reaction, and therefore called deactivating groups.
