Question
Question: In the reaction A is: 
A. H3PO2
B. Cu2Cl2
C. HgSO4/H2SO4
D. H+/H2O
Solution
We need to know that in natural science, the reaction between arene diazonium salts with fragrant amino mixes in the acidic medium (or) reaction between arene diazonium salts with phenol in essential medium outcomes in the development of azo mixes that are splendidly hued.
Complete step by step answer:
We have to remember that the diazonium salts are integrated by the expansion of cold arrangement of sodium nitrite to arylamine arrangement in weakening corrosive at a temperature not exactly. This cycle is known as diazotization. The reaction of the benzenediazonium chloride with another compound involving a benzene ring known as coupling specialist like phenol (or) fragrant amine prompts the development of the azo compound. A few of the results of the coupling reaction are significant colors. A colored precipitate of an azo compound is delivered when the reaction of the diazonium salt with amines (or) phenols.
The given reaction is:
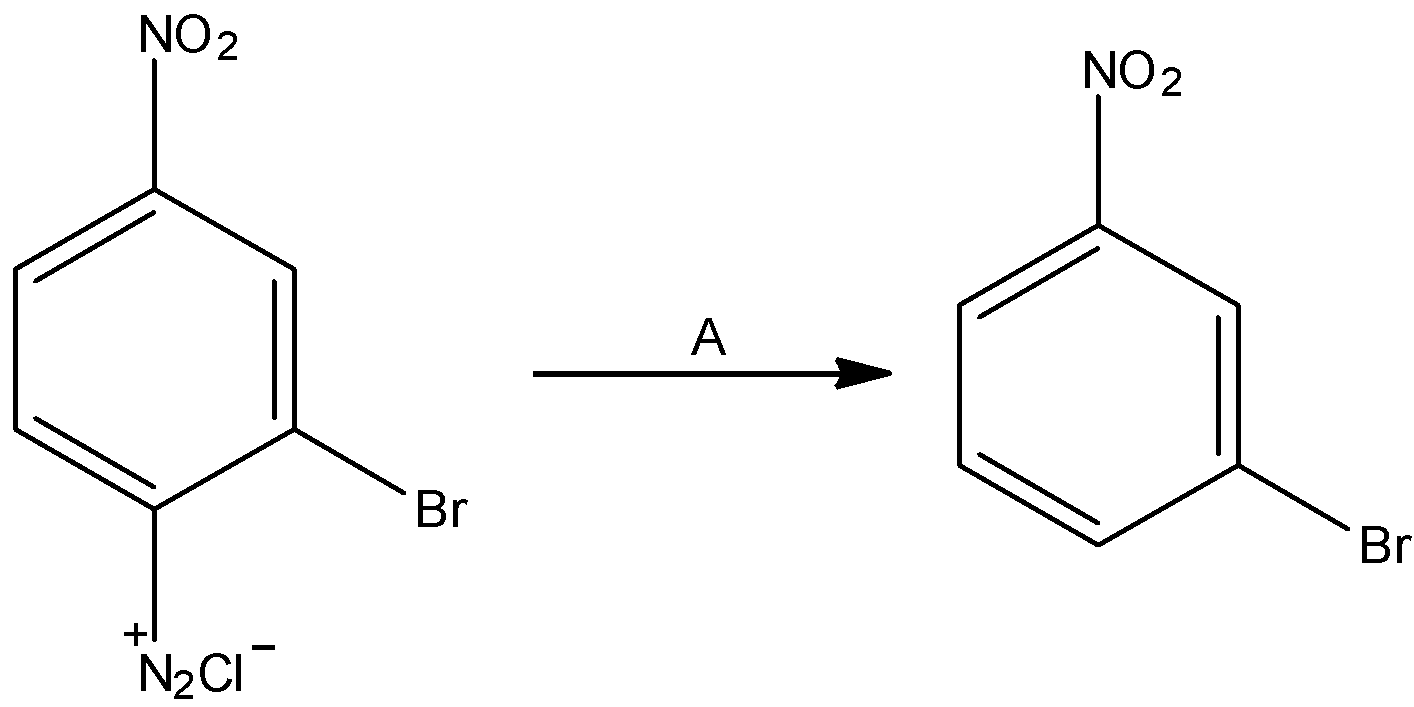
The diazonium group is supplanted with hydrogen molecules.
This is an advantageous strategy to eliminate an aromatic amino group in a two-step method.
The initial step is the diazotization which changes the aromatic amino group over to the diazo group and the subsequent reaction is the substitution of a diazo group by an atom of hydrogen.
In the reaction, we can see that benzene diazonium salt results in the formation of 1-bromo-3-nitrobenzene. The chemical compound is identified as H3PO2
So, the correct answer is Option A.
Note: We need to remember that the azo dyes are generally utilized in textile, fiber, cosmetic, calfskin, paint, and printing enterprises. Other than their trademark shading capacity, azo compounds are accounted for as antibacterial, antiviral, antifungal, and cytotoxic specialists. Examples of few azo compounds which are used as pH indicators are - Methyl orange, Methyl yellow, Methyl red, Congo red, Alizarin yellow. Examples of dyes that contain azo linkages areAniline yellow, Alizarin yellow R, Bismarck brown Y, and Congo red.
