Question
Question: In the reaction, \(2X + {B_2}{H_6} \to {\left[ {B{H_2}{{\left( X \right)}_2}} \right]^ + }B{H_4}^ - ...
In the reaction, 2X+B2H6→[BH2(X)2]+BH4−, the amine (s) X is (are):
A. NH3
B. CH3NH2
C. (CH3)2NH
D. (CH3)3N
Solution
We have to know that smaller amines on reaction with diborane gives diammonium salt but larger amines produce adduct.
Complete step by step answer:
We know that amines are basic chemical derivatives of ammonia in which the attached hydrogens are replaced by alkyl or aryl groups. We can classify amines into,
Primary amine: One organic group attached to nitrogen atoms.
Secondary amine: Two organic groups bonded to nitrogen atoms.
Tertiary amine: Three organic groups bonded to nitrogen atoms.
An example of primary amine is CH3NH2.
An example of secondary amine is (CH3)2NH.
An example of tertiary amine is (CH3)3N.
We can call NH3, CH3NH2, (CH3)2NH as smaller amines, whereas (CH3)3N is a tertiary amine.
When smaller amines like NH3, CH3NH2, (CH3)2NH are reacted with diborane, an unsymmetrical cleavage of diborane occurs and the product formed will be an ionic compound. We can write the general reaction as,
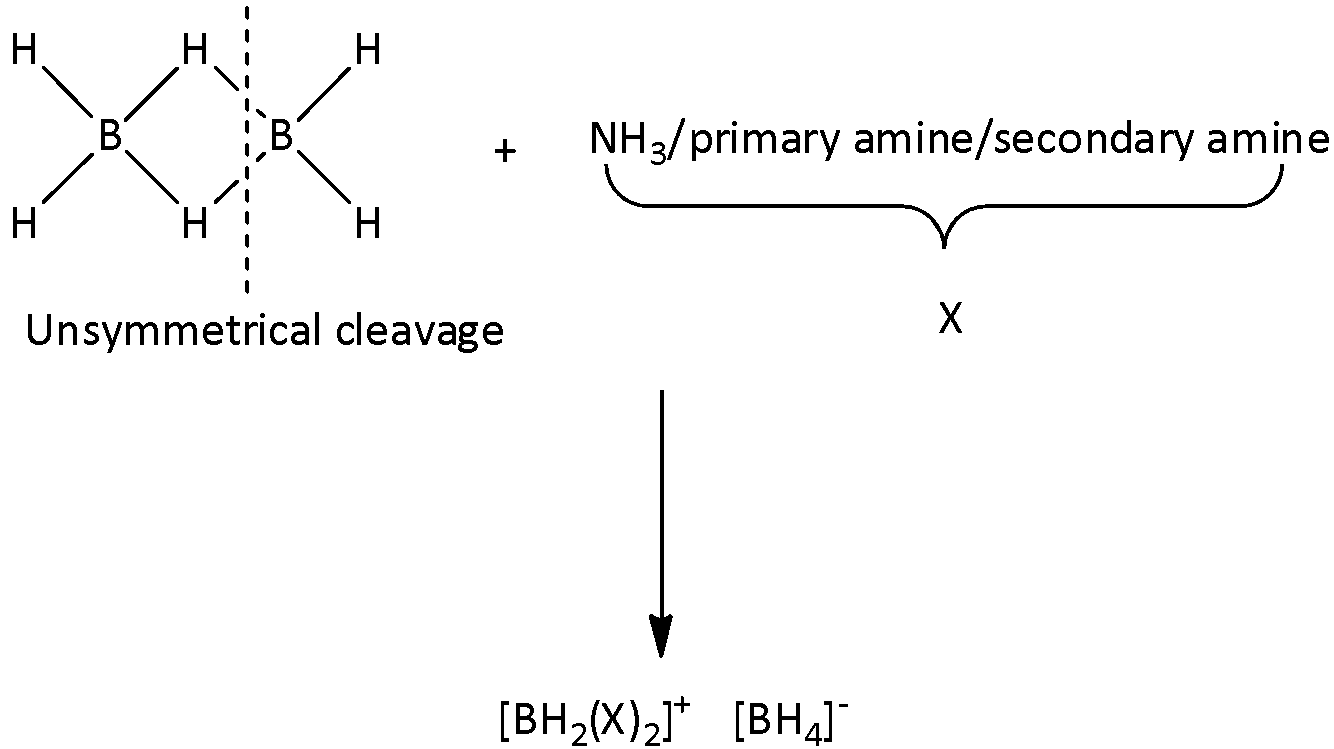
.
Whereas, when larger amines like (CH3)3N are reacted with diborane, asymmetric cleavage of diborane occurs and adduct is formed as product. We can write the general reaction as,
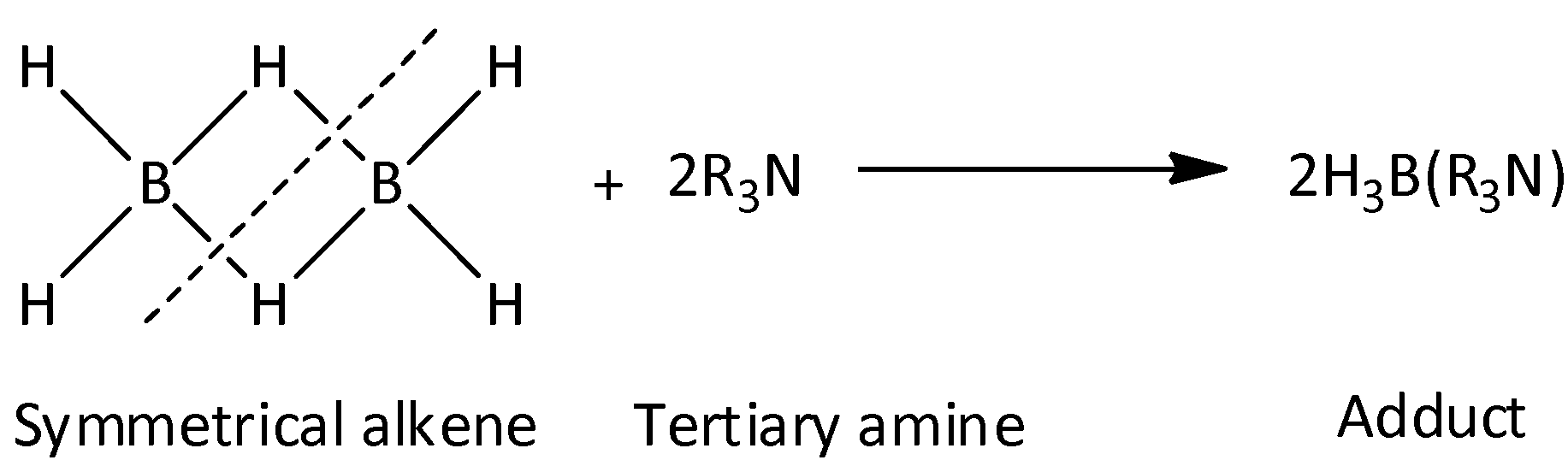
When NH3 is reacted with diborane, the product formed would be [BH2(NH3)2]+[BH4]−. We can write the reaction as,
2NH3+B2H6→[BH2(NH3)2]+BH4−
Therefore, the option (A) is correct.
When CH3NH2 is reacted with diborane, the product formed would be [BH2(CH3NH2)2]+[BH4]−. We can write the reaction as,
2CH3NH2+B2H6→[BH2(CH3NH2)2]+BH4−
Therefore, the option (B) is correct.
When (CH3)2NH is reacted with diborane, the product formed would be [BH2((CH3)2NH)2]+[BH4]−. We can write the reaction as,
2(CH3)2NH+B2H6→[BH2((CH3)2NH)2]+[BH4]−
Therefore, the option (C) is correct.
When (CH3)3N is reacted with a diborane, the product formed would be adducted. We can write the reaction as,
2(CH3)3N+B2H6→2[(CH3)3N→BH3]
Therefore, the option (D) is incorrect.
So, the correct answer is Option A,B,C.
Note:
As we know that primary amines have the highest melting points as they have polar nitrogen-hydrogen bonds present in them.
Secondary amines are said to have lower melting points than primary amines because the N−H bond of a secondary amine is less polar than that of primary amine, the dipole-dipole attractions between the secondary amine molecules are lower. This is reflected in the lower melting points.
Tertiary amines have the least melting point because they do not have a N−H bond, they cannot form any intermolecular hydrogen bonds with other tertiary amines, and this contributes to the least melting point of tertiary amine.
Diborane can be used as propellant in a rocket, used in production of borophosphosilicate, used as a reducing agent. It could act as catalyst and as rubber vulcanizer in polymerization reactions. It is used as a doping agent in manufacturing semiconductor devices.
