Question
Question: In the purification of copper, the setup of the electrolytic cell is shown in the given figure. Choo...
In the purification of copper, the setup of the electrolytic cell is shown in the given figure. Choose the correct options related to this setup. The negative ion P is :
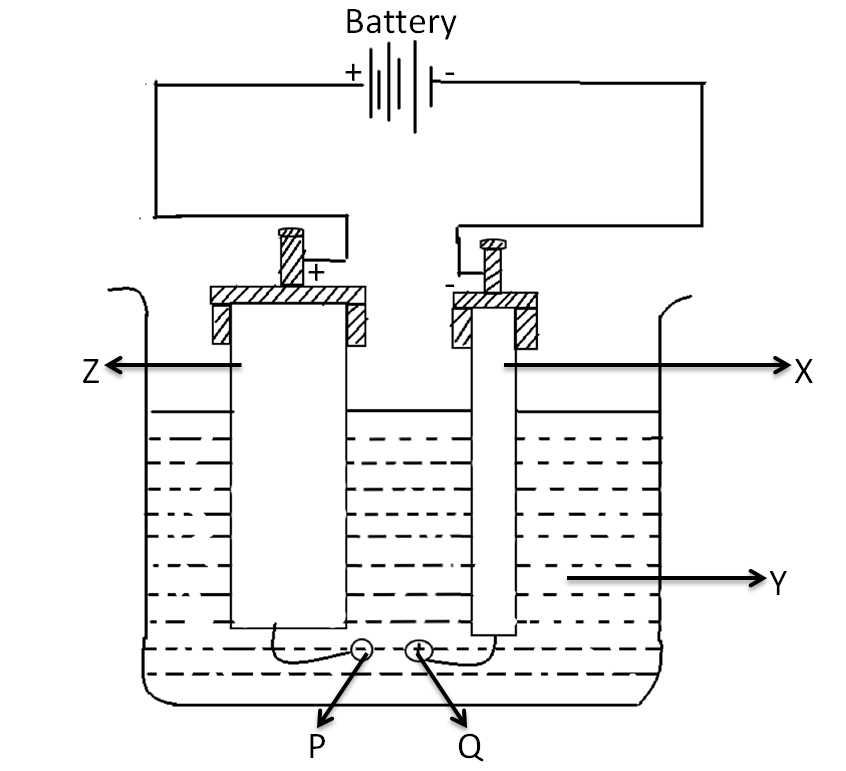
(A)- SO42−
(B)- NO3−
(C)- Cl−
(D)- I−
Solution
A technique which uses the application of electric current of a suitable voltage for the extraction, as well as for the purification of metals obtained by any refining method is known as Electrolysis or Electrolytic refining.
Complete Step by step solution:
-In the process of electrolysis, the impure metal acts as an anode and the pure metal act as a cathode. A solution of a soluble salt of the same metal is prepared and taken as an electrolyte. When the electricity is passed through the electrolytic cell, the metal ions from the electrolyte are deposited at the cathode as the pure metal from the anode dissolves in the electrolyte in the form of ions. The impurities present in the impure metal now get collected below the anode and this anode is now known as anode mud, and thus the pure metal is separated from its impurities.
-The extraction of pure copper from copper ores through the process of electrolysis involves the deposition of copper on the cathode, using lead-coated plates as an anode. The purification of copper involves the use of copper sulphate solution as the electrolyte, along with high purity copper strips as the cathode and impure copper as anodes.
-So the pure copper plate is the cathode, that is X and the impure copper plate is the anode, that is Z. Therefore, the negative terminal of the battery should be connected to the cathode of the electrolytic cell, that is the pure copper plate, labelled as A in the figure and the anode is connected to the anode of the electrolytic cell, that is the impure copper plate labelled as B. The labelling Y is indicating the salt solution of copper sulphate.
During the electrolysis process, the copper from the impure plate is deposited onto the pure plate copper through the electrolytic copper sulphate solution, hence the negative ion P is SO42−
So, the correct answer is option A.
Note: Following are the other two methods other than the electroplating method for the purification of metals –
(i) Zone refining- The idea that the impurities are more soluble in the molten state of metal than the metal in the solid-state forms the principle of the Zone refining process. In the zone refining process, the rod of impure metal is fixed in a circular mobile heater. As the mobile heater moves, the molten zone of the rod also moves and starts melting. The impurities are passed to the adjacent molten zone. The impurities are segregated at one end of the rod and the process is repeated till all the impurities are separated. Metals such as germanium, silicon, gallium, indium, and boron are refined with this method.
(ii) Vapour phase refining- For this type of refining process, the metal should form a volatile compound in the presence of a reagent and it should be easily decomposed to recover the metal. Metals like nickel, zirconium, and titanium are refined using this method.
