Question
Question: In the plot of \(\Lambda \)and\(\sqrt{C}\), the slope is 1)\({{\Lambda }^{0}}\) 2) –b 3) \(\d...
In the plot of ΛandC, the slope is
1)Λ0
2) –b
3) R−2.303
4) ∞
Solution
Λis the notation used for conductance and the answer lies in the equation called Debye-Huckel-Onsager’s equation which has the formula Λc= Λ0 - bCwhere in slope of this equation gives the answer.
Complete answer:
We have learnt in the basic chapters of chemistry that conductivity of ions in dilute solutions of strong electrolytes is derived on the basis of Debye-Huckel theory which is in turn derived from Kohlrausch’s law.
This is given by the formula,
Λc= Λ0 - bC ……..(1)
where, Λc= initial conductance
Λ0= conductance at the infinite dilution
b = constant and C= concentration
Now, if we compare equation number (1) with the basic equation for a straight line which is given by,
y=mx+c …(2)
In this c = intercept and m = slope
Therefore on comparison of the above formula with that of equation (1), we can say that intercept c = Λ0 and slope can be found by plotting a graph.
Equation number (2) in the form of graph can be plotted as y v/s x.
Similarly equation (1) can be plotted as shown below,
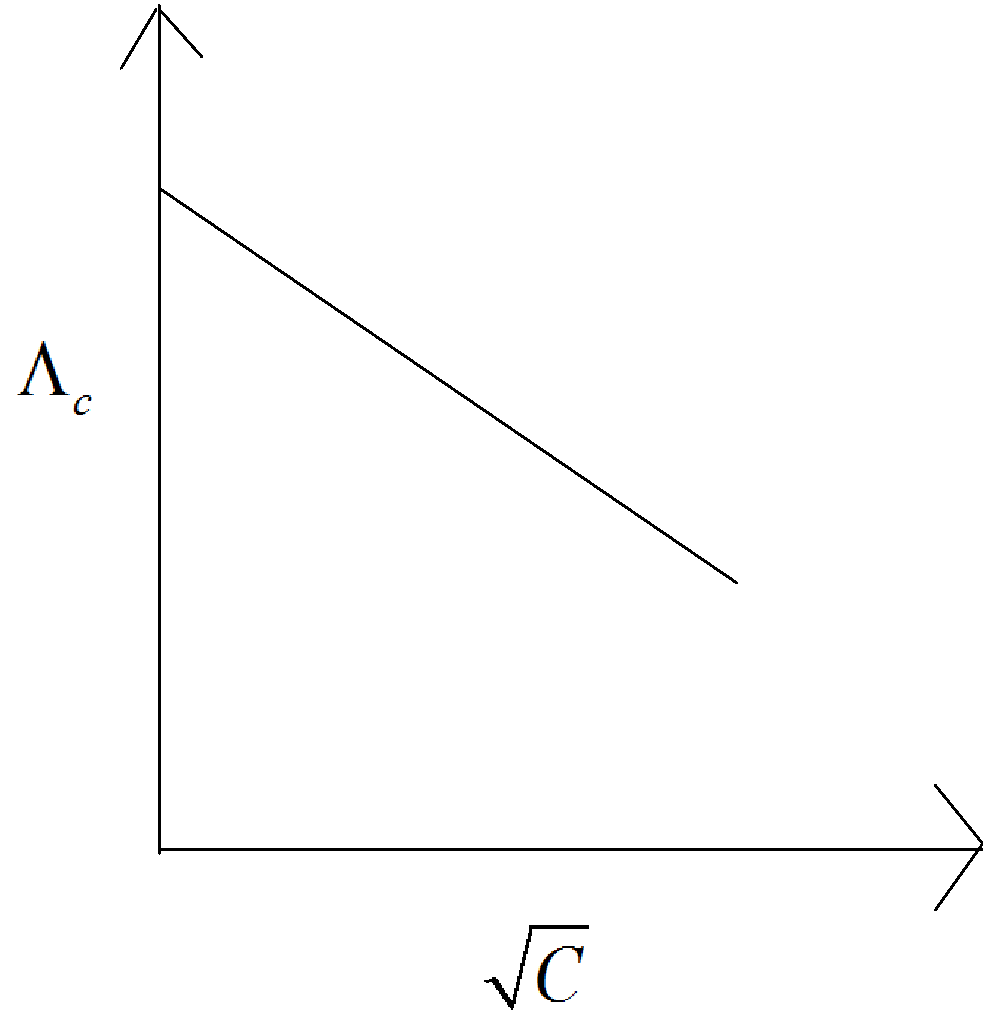
Here from the graph shown above, we can see that the slope is negative, that is the line is decreasing from top to bottom.
Therefore, slope is given by m and by comparison of equation numbers (1) and (2) we can get the slope as m = -b
Thus, the correct answer will be option (b) –b.
Note:
The equation of Kohlrausch’s law and that of Debye-Huckel-Onsager theory are two different equations with a slight change in the quantities. Therefore, note that Onsager gave a theoretical explanation of Kohlrausch’s law by the extension of Debye-Huckel theory.
