Question
Question: In the \[\;P - V\] diagram shown, the gas does \[5J\] of work in isothermal process \[\;a - b\] and ...
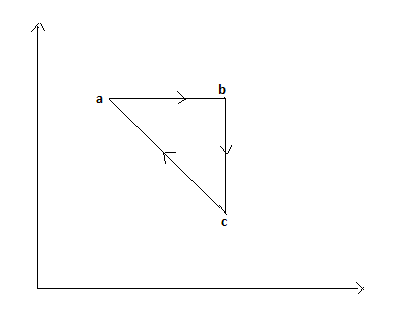
In the P−V diagram shown, the gas does 5J of work in isothermal process a−b and 4J in adiabatic process b−c. What will be the change in internal energy of the gas in the straight path c toa?
A) (A)9J
B) (B)1J
C) (C)4J
D) (D)5J
Solution
The isothermal process is the process in which the temperature of the system remains constant when converting one form of energy into another form. It can also be said that the work is done in the isothermal process by keeping the temperature constant. Contrastingly, in the adiabatic process, the temperature is varying to keep the heat in a constant value.
Complete step by step answer:
From the diagram, we can understand that the process is cyclic. Therefore the net change in internal energy between the processes a to b, b to c and cequals to zero.
∴ΔU=ΔUa+ΔUb+ΔUc=0......(1)
Since the process a−b is isothermal, the temperature is constant. Therefore the change in internal energy will be zero.
∴ΔUa=0
And the process b−c is adiabatic, therefore the heat is constant.
∴ΔQ=0
Hence from the equation (1),
ΔUa+ΔUb+ΔUc=0
→ΔUb=−ΔUc.....(2) (As we know ΔUa=0)
The internal energy decreases when the work is done using that energy ΔUb=−ΔW.
As we know that the work is done in the process b−c is 4J
→ΔUb=−4J
Applying this value in the equation (2), we get
ΔUc=4J
Thus the change in internal energy of the gas in the straight path c to a is 4J.
Hence the correct option is C.
Note: The difference between the adiabatic and the isothermal process is that the heat is constant in the adiabatic process and the temperature is constant in the isothermal process.
The isothermal process occurs slowly to keep the equilibrium temperature.
Boyle’s law gives the relation between the pressure and volume (P−V) for the ideal gas of an object. It states that the pressure exerted by the object of an ideal gas is inversely proportional to its volume when the temperature and the amount of gas remain unchanged in the system. That is we write it as, PV=k
