Question
Question: In the multi-step conversion of an aldose into next higher aldose by Killani-Fischer synthesis, the ...
In the multi-step conversion of an aldose into next higher aldose by Killani-Fischer synthesis, the reagent employed in the first step is NH2OH
A. True.
B. False.
Solution
We know that Killani-Fischer synthesis is the method in which monosaccharides are prepared. In this method we have to increase the number of carbon atoms in the carbon chain so the reagent used should be in the way that it should raise the number of carbon atoms.
Complete step by step answer:
We must remember that an aldose sugars are monosaccharides with carbon chain backbone which have a carbonyl group at the last carbon atom which means that it is an aldehyde on the other hand; hydroxyl groups are connected to the rest of the carbon atoms in the molecule.
Example: Glucose.
In the initial step NaCN/H2O is taken as reagent. We can write a chemical reaction of this type as,
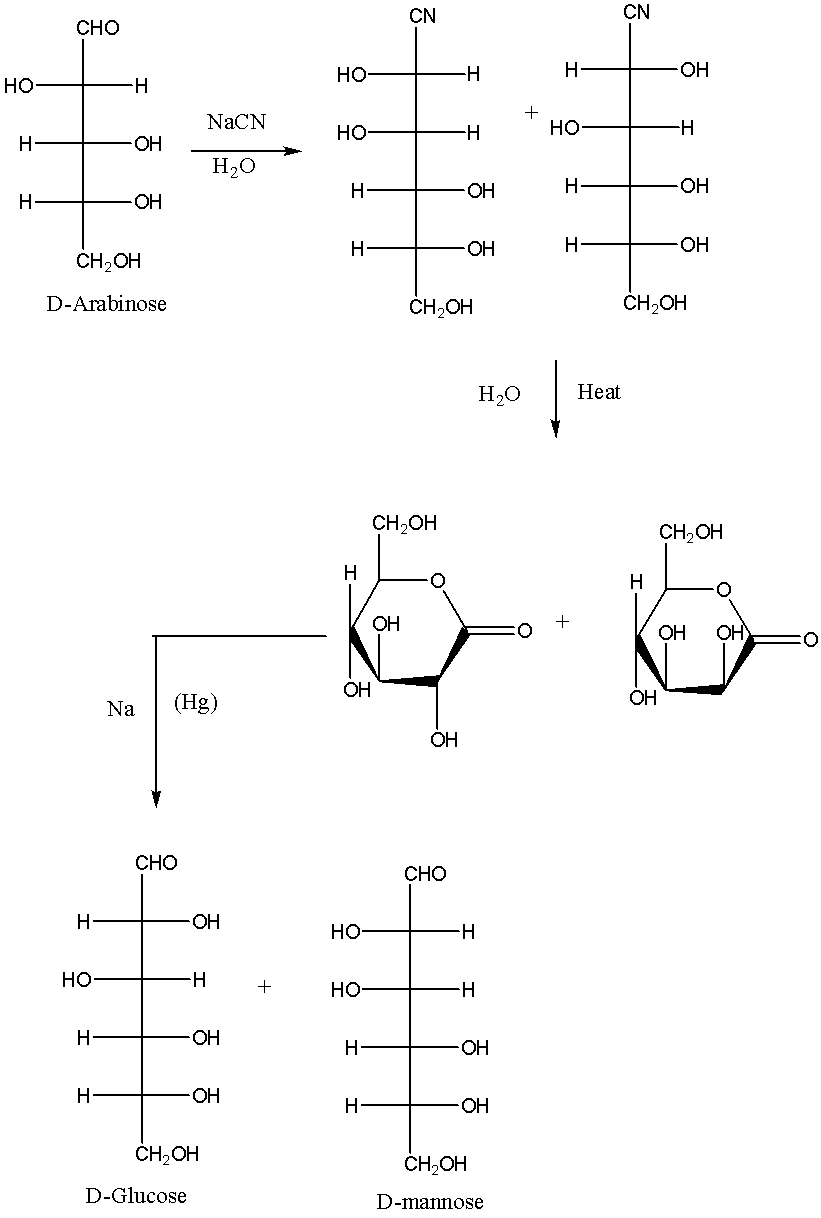
Therefore, the statement is false.
Note: Killani–Fischer synthesis:
We need to know that the Killani–Fischer synthesis proceeds via cyanohydrin and aldonic acid lactone intermediates. The main step is the reaction of the preliminary sugar with aqueous cyanide. The cyanide acts as nucleophile and add to the group of the sugar (whereas sugars tend to exist mostly as cyclic hemiacetal, they're always in balance with their open-chain aldehyde or ketone forms, and within the case of those aldose it's that aldehyde form that reacts during this synthesis). The cyanohydrin resulting from this addition is then heated with water, which on hydrolysis the cyanide is converted into an acid group that rapidly reacts with itself to make a more stable lactone. Now there are two diastereomeric lactones within the reaction mixture. They’re separated (by chromatography, partition into different solvents, or any of the various other separation methods) then the specified lactone is reduced with a sodium amalgam.
