Question
Question: In the Lewis structure of \(S{O_3}\). What is the formal charge on the atom \(O\)?...
In the Lewis structure of SO3. What is the formal charge on the atom O?
Solution
The Lewis structure is also called an electron dot structure which determines the number of valence electrons present in an atom. Moreover, they also describe how these valence electrons are participating in the bond formation to form a molecule.
Complete answer:
According to octet rule, the maximum number of electrons that can be filled within a valence shell is eight. When there are less than eight electrons only then an atom undergoes a bond formation either by accepting or donating electrons to achieve a stable condition like noble gases.
The sulfur trioxide is a tetra atomic chemical molecule where both the sulfur and three oxygen molecules bond with an equal number of valence electrons. The total number of valence electrons in a single sulfur trioxide molecule is 24. Six electrons are needed to complete the octet in the SO3 molecule, where both sulfur and oxygen atoms need two valence electrons to stabilize their atom.
In the molecule of sulfur trioxide there are three double covalent bond between sulfur and oxygen atom each. The structure of sulfur trioxide is trigonal pyramidal, as the double bond is formed there are no lone pair exists on the central atom sulfur. Sulfur trioxide does not have a charge and the oxygen atoms are so electronegative that sulfur surrenders its electron to them, because sulfur is weak compared to oxygen.
To calculate formal charge on oxygen, using the following equation:
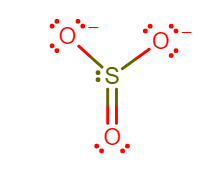
Formal charge= (Number of valence electrons in free atom) − (Number of Lone-pair electrons) − (21 number of pair electrons)
Formal charge on Oxygen atom= (6)−(6)−(21×2)
Therefore, formal charge on oxygen is −1
Note:
The common arrangement of oxygen that has formal charge of zero is when the oxygen atom has two bonds and two lone pairs. Oxygen can also exist as a radical, such as where an oxygen atom has one bond, two lone pairs, and one unpaired electron, giving it a formal charge of zero.
