Question
Question: In the given wave function, $\psi = \frac{1}{81} (\frac{2}{\pi})^{\frac{1}{2}} (\frac{1}{a_0})^{\fra...
In the given wave function, ψ=811(π2)21(a01)23(6−a0r−a02r2)e−3a0rcosθ
Identify the correct choice(s).
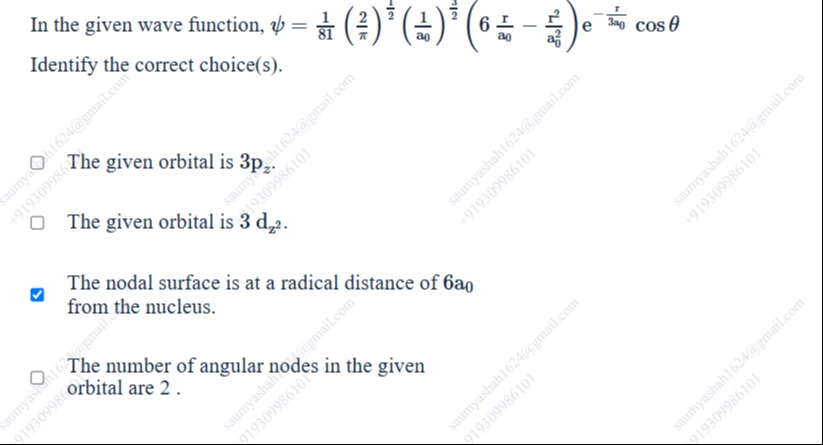
The given orbital is 3pz.
The given orbital is 3dz2.
The nodal surface is at a radical distance of 6a0 from the nucleus.
The number of angular nodes in the given orbital are 2.
The nodal surface is at a radical distance of 6a0 from the nucleus.
Solution
Solution:
The given wave function is
ψ=811(π2)1/2(a01)3/2(6−a0r−a02r2)e−3a0rcosθ.-
Angular Part:
The factor cosθ is the angular part. For hydrogen-like orbitals, cosθ corresponds to the spherical harmonic Y10(θ,ϕ) which represents a pz orbital (with l=1). In contrast, a 3dz2 orbital would have an angular part proportional to (3cos2θ−1). -
Radial Part and Radial Node:
The radial part is given by
Setting the polynomial equal to zero to find the radial node:
6−a0r−a02r2=0.Multiply by a02:
r2+ra0−6a02=0.Let x=a0r; then
x2+x−6=0.Solving, we get:
x=2−1±1+24=2−1±5.The positive root is:
x=2⇒r=2a0.So the radial node is at 2a0 and not at 6a0.
- Angular Nodes:
Since cosθ vanishes at θ=π/2, there is 1 angular node. (For l=1 there is exactly one angular node.)
