Question
Question: In the given structure, number of sp and sp2 hybridized carbon atoms present respectively are:...
In the given structure, number of sp and sp2 hybridized carbon atoms present respectively are:
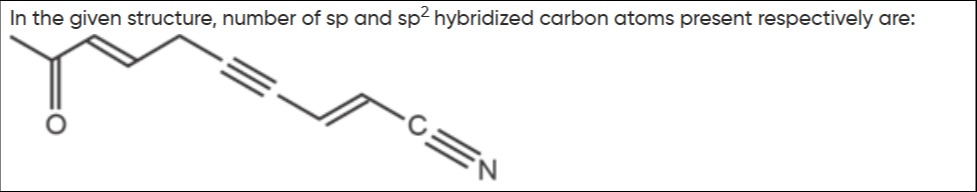
A
3 and 5
B
2 and 5
C
3 and 3
D
2 and 3
Answer
3 and 5
Explanation
Solution
To determine hybridization, count sigma and pi bonds: 4 sigma = sp³, 3 sigma + 1 pi = sp², 2 sigma + 2 pi = sp.
Analyzing the structure CH₃-C(=O)-CH=CH-CH₂-C≡C-CH=C-C≡N:
- C1 (CH₃): sp³ (4 single bonds)
- C2 (C=O): sp² (1 double bond, 2 single bonds)
- C3 (CH=): sp² (1 double bond, 2 single bonds)
- C4 (=CH-): sp² (1 double bond, 2 single bonds)
- C5 (CH₂): sp³ (4 single bonds)
- C6 (C≡): sp (1 single bond, 1 triple bond)
- C7 (≡C-): sp (1 triple bond, 1 single bond)
- C8 (CH=): sp² (1 double bond, 2 single bonds)
- C9 (=C-): sp² (1 double bond, 2 single bonds, implies one H)
- C10 (C≡N): sp (1 single bond, 1 triple bond)
sp hybridized carbons: C6, C7, C10 (3) sp² hybridized carbons: C2, C3, C4, C8, C9 (5) sp³ hybridized carbons: C1, C5 (2)
Therefore, there are 3 sp and 5 sp² hybridized carbon atoms.
