Question
Question: In the given reactions: Which of the following statements are correct about the given reactions? ...
In the given reactions:
Which of the following statements are correct about the given reactions?
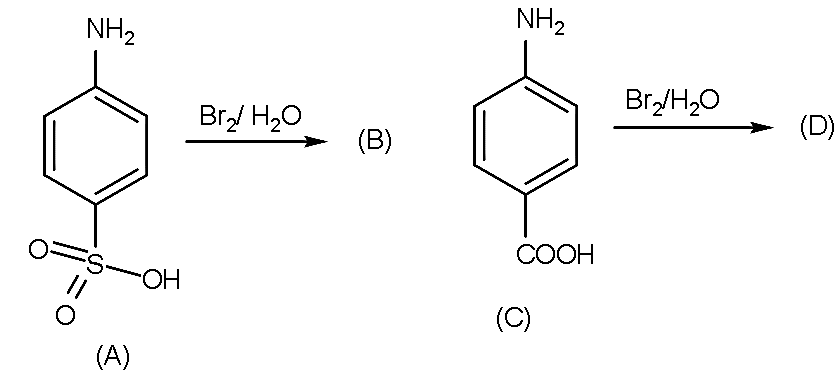
A. (B) and (D) are the same products as given.
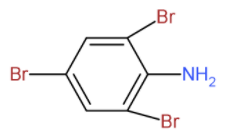
B. (B) is as shown in figure
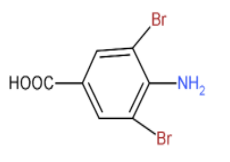
C. (D) is as shown in the figure.
D. The above reaction is called ipso substitution.
Solution
For this problem, we have to study the effect of bromine on the given reactant which will undergo the electrophilic aromatic substitution reaction. Bromine attaches on the meta position only whereas amino groups attach on the ortho or para position only.
Complete step by step solution:
- In the given question, we have to explain the correct product which will be formed by the two aromatic compounds that will react with bromine.
- As we know that electrophilic aromatic substitution reaction is a type of reaction which usually takes place in the aromatic compounds in which the electrophile is responsible for replacing the functional group from the meta position.
- The electrophile replaces the functional groups from meta-position because they are highly meta directing elements and most stable there.
- So, in the first reaction the bromine will replace the sulphonic acid along with it, bromine will also join on the ortho position from the amino group.
- The reaction is shown below:
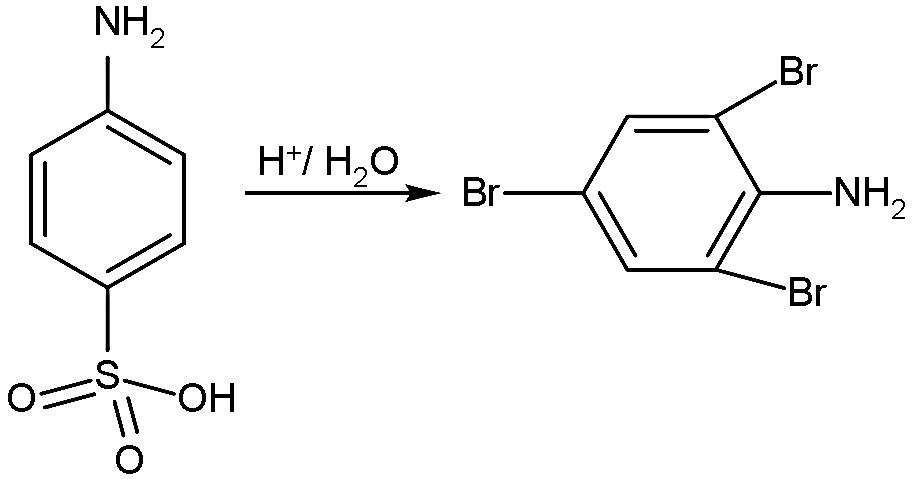
- Similarly, with the second reactant, the carboxylic acid will also be replaced by the bromine at the meta position and also it is introduced on the ortho position to the amine group:
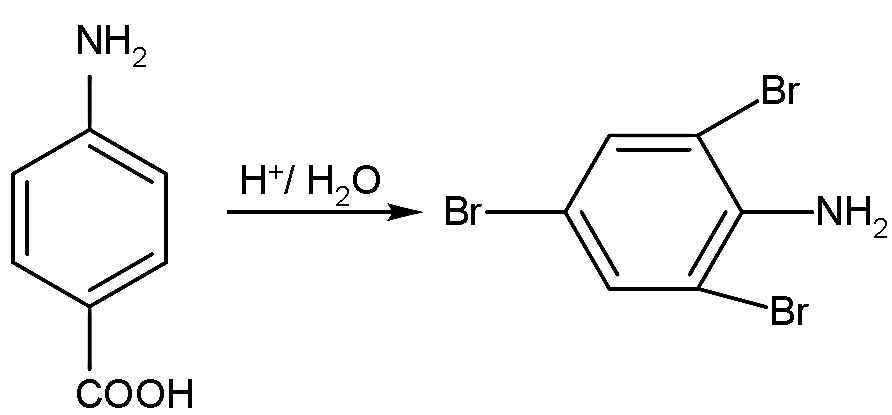
- Now, these reactions are also ipso substitution reactions, the reaction in which one or more substituent in the aromatic ring is replaced by an electrophile.
Therefore, option A and D are the correct answer.
Note: The electrophile is a species which are electron-deficient and can accept a pair of electrons from the electron donor species which are also known as neutrophils. In this question, bromine is an electrophile.
