Question
Question: In the given reactions, the rate of reaction of (I) and (II) are the same. Both reactions proceed by...
In the given reactions, the rate of reaction of (I) and (II) are the same. Both reactions proceed by which mechanism?
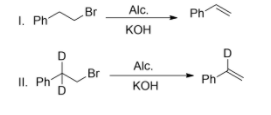
(A)- E1
(B)- E2
(C)- E1cb
(D)- Anti-elimination
Solution
The type of organic reaction in which two substituents are removed from a molecule in either a one-step mechanism or two-step mechanism is known as an elimination reaction. β-elimination is a chemical reaction in which atoms or groups are lost from adjacent atoms resulting in a new pi-bond formation, usually between two carbon atoms. The atoms lost is usually a proton, but not always.
Complete Step by step solution:
-When the reaction follows a one-step mechanism for elimination reaction, such reactions are said to be E2reaction. When the reaction follows a two-step mechanism for elimination reaction, such reactions are said to be E1reactions.
-The number 1 and 2 in E1and E2does not refers to the number of steps in the mechanisms rather refers to the kinetics of the reaction, that is E2is a bimolecular (second-order) reaction while E1is a unimolecular (first-order) reaction.
-E1cb exists where the molecule can stabilize an anion but possesses a poor leaving group.
-Following the E1mechanism, carbocation is formed from the heterolytic cleavage of the C-Br bond. This is a slow step, hence is the rate-determining step. The proton abstraction now occurs from the adjacent carbon resulting in the formation of an alkene. Thus, only halide affects the rate of reaction.
-Following E2mechanism, both Br atom and Deuterium or Hydrogen atom leave simultaneously and thus affect the rate.
-Following E1cb, the first step is the removal of proton resulting in carbanion formation followed by the cleavage of halide or leaving group. Since, E1cb is a two-step mechanism, an isotope effect will be present.
-Anti-elimination and E2eliminations are the same.
Therefore the correct answer is option A.
Note: The reactivity of halogens, iodide, and bromine are being favoured for influencing the rate of reaction. Fluoride not being a good leaving group hence gives elimination reactions at slower rates than other halogens. There is a competition between the elimination reaction and nucleophilic substitution reactions, E2 and SN2, and E1 and SN1reactions. Substitution reactions generally dominate and elimination occurs only during precise circumstances. Elimination reactions are generally favored over substitution reactions when
(i) steric hindrance around the α-carbon increase.
(ii) a stronger base is used.
(iii) increase in temperature.
(iv) the base is a poor nucleophile.
