Question
Question: In the given reaction, what is product B? 
Solution
Treatment of aromatic alkanes oxidation KMnO4 to give carboxylic acids. The position in the benzene ring directly adjacent to an aromatic group is known as the benzylic position. The oxidation of aromatic alkanes takes place only when there is hydrogen attached to the carbon.
Complete answer:
There are two products A and B in the given reaction. so this reaction is divided into two reactions.
(i)
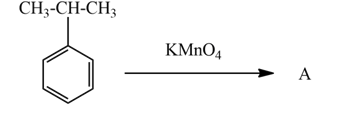
The above reaction is the oxidation of aromatic alkane which is cumene by KMnO4and gives benzoic acid. Cumene contains secondary carbon influences by potassium permanganate cleaving the chain and leaving one C forms –COOH functional group. In this reaction KMnO4acts as an oxidizing agent.
Hence, the product A is benzoic acid.
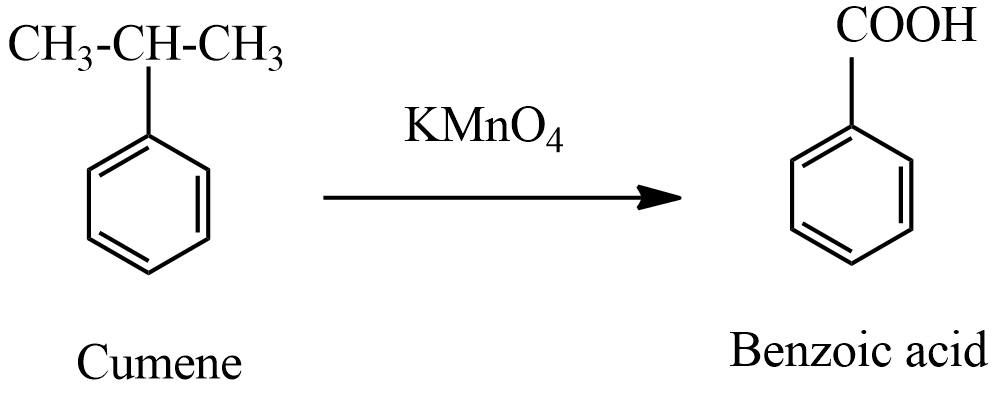
(ii) product A which is formed in the first step is benzoic acid. When this benzoic acid is heated at 630oC with soda-lime benzene compound is formed.
This reaction of soda-lime is treated with alkanes formation of sodium salts of alkanes, further reaction gives another alkane is a type of decarboxylation reaction. In this reaction, benzoic acid treated with soda lime forms benzene along with sodium carbonate belongs to the decarboxylation reaction.
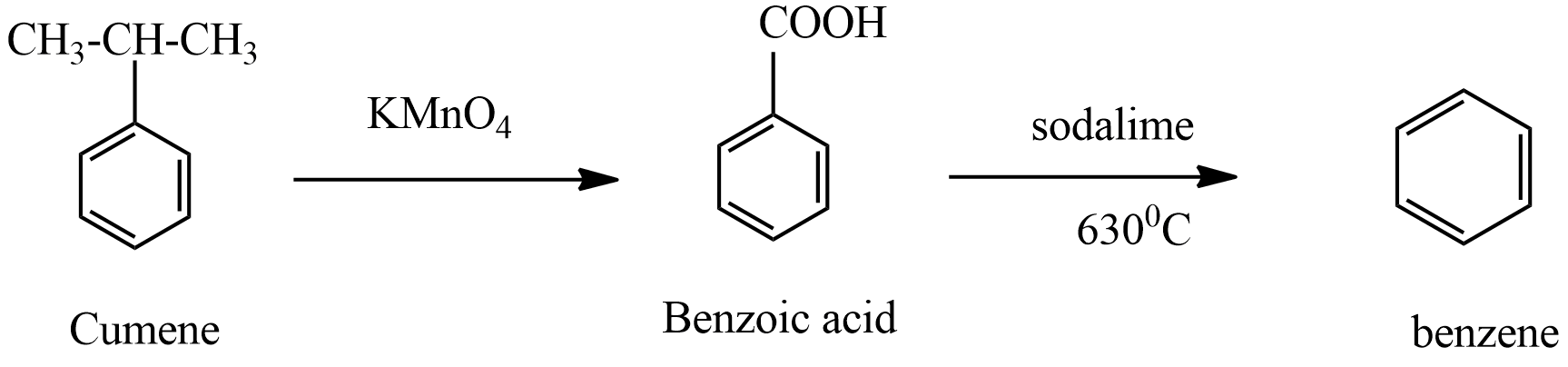
Hence, product B in the given reaction is Benzene.
The correct answer is option C.
Note:
The mixture of NaOH and CaO is known as soda-lime which is used in granular form in closed breathing environments, like general anesthesia, submarines, and recompression chambers. Soda-lime is used to remove carbon dioxide from breathing gases to prevent carbon dioxide retention and 19% of its weight of carbon dioxide absorbed by soda lime.
