Question
Question: In the given reaction, the final compound (L) is:  is:
Solution
The given reaction proceeds in three steps. In the first step carboxylic acid is converted to ester by substitution reaction. In the second step Grignard reagents on reacting with ester forms and intermediate which further on acid hydrolysis gives alcohol.
Complete step by step answer:
This reaction is taking place usually in three step, in first step 2-nitrobenzoic acid is reacted with ethanol in acidic medium to second step 2-nitrobenzoate is reacted with excess of Grignard reagent to form an intermediate. In the third step the intermediate is reacted with hydronium ion gives the final product (2-nitrophenyl)diphenylmethanol as a final product.
The reaction is shown below.
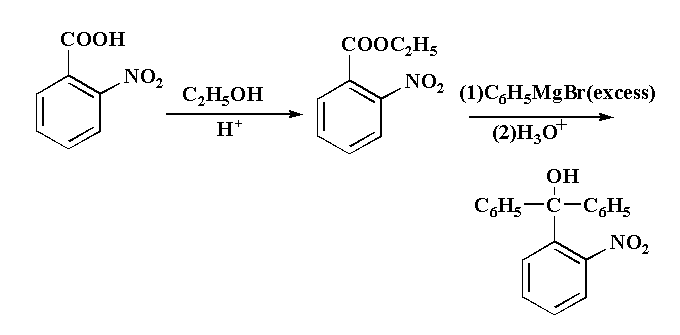
The mechanism of the reaction is that, in the given reaction, first the lone pairs present in the oxygen atom of ethanol attack the hydrogen ion from the acidic medium to form C2H5O+H2, then removal of water takes place which result in the formation of CH3−CH2+. The lone pair present in the oxygen of the hydroxyl group attached to the carbon of the carboxylic group of 2-nitrobenzoic acid attack the carbocation CH3−CH2+ to form 2-nitrobenzoate which is an ester. The compound 2-nitrobenzoate is then reacted with benzyl magnesium bromide (Grignard reagent) where the C6H5− attack the carbonyl carbon and gets attached to it by removing C2H5COMgBr and then the negative oxygen ion attack the hydronium ion to form the final product (2 nitrophenyl)diphenylmethanol.
The final product L is (2 nitrophenyl)diphenylmethanol.
Note:
In the final product, you can see that two phenyl groups are attached to the carbon, this is because phenyl magnesium bromide (Grignard reagent) is present in excess quantity so it is added twice in the reaction.
