Question
Question: In the given Dewar structure of benzene, which of the following statement(s), is correct? , is correct?
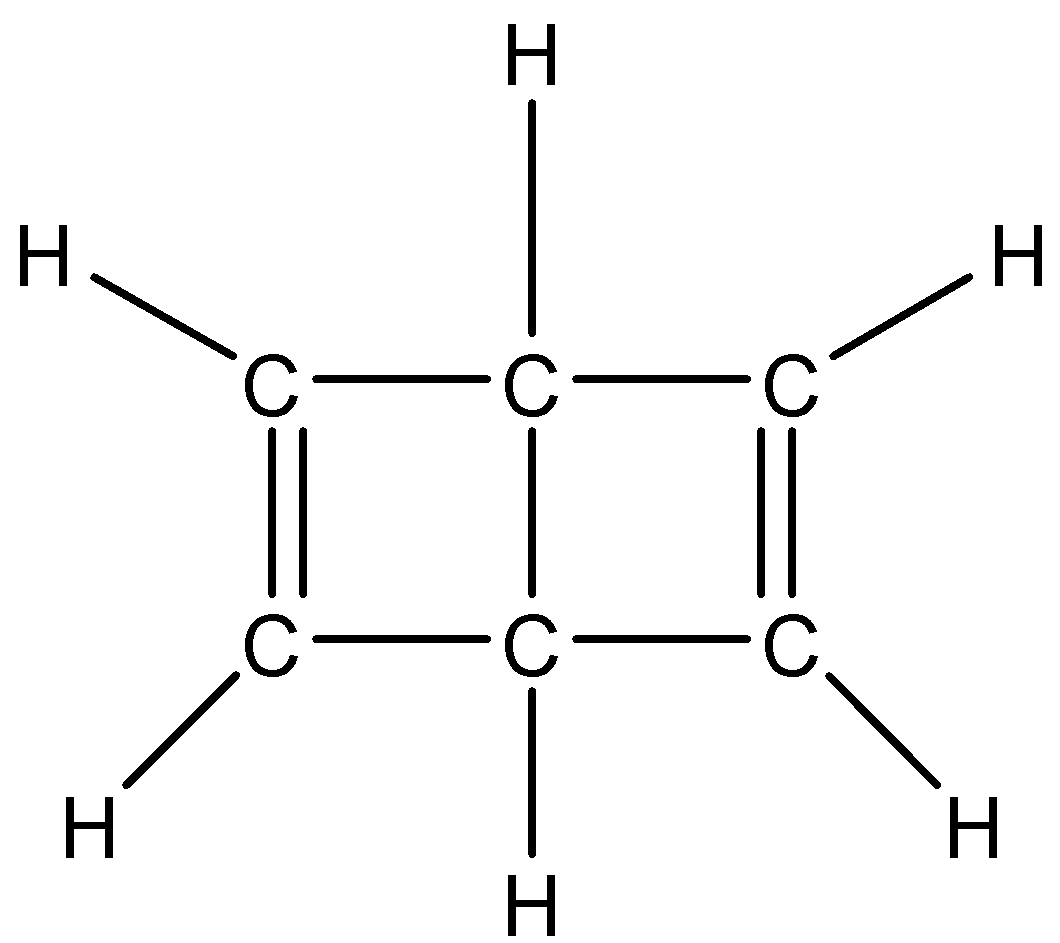
(A) All the carbons are in sp2 hybrid state
(B) All the carbons are in sp3 hybrid state
(C) Four carbons are in sp2 and two in sp3 hybrid state
(D) Four carbons are in sp3 and two in sp2 hybrid state
Solution
In the given options we are informed about the hybridization state of carbons in the molecule, so we have to configure the correct hybridization state of every Carbon atom. Hybridization is basically the mixing of atomic orbitals to form new types of orbitals.
Complete answer:
Hybridization is the phenomenon in which atomic orbitals that have similar energy are combined together to make new atomic orbitals with different structures and properties. The new orbitals that are formed after the mixing are called ‘Hybrid Orbitals’. Based on mixing hybridization is divided into 6 types, these are sp,sp2,sp3,sp3d,sp3d2,d2sp3.
Carbon atom shows only three types of Hybridization i.e. sp,sp2,sp3. The sp hybridization of carbon is shown when it is bonded with two atoms with 2double bonds or it is bonded with 1 single and 1 triple bonds. The type of arrangement in molecules with sp hybridization is linear with a bond angle of 1800.
Carbon shows sp2hybridisation when there is a mixing of 1s orbital and two 2p orbitals. In this carbon forms two single bonds and one double bond with a total three atoms. The orbitals have a triangular geometry with a bond angle of 1200.
Carbon shows sp3 hybridisation when there is a mixing of 1s orbital and three 3p orbitals. In this carbon forms four single bonds with four atoms. The orbitals have a tetrahedral geometry with a bond angle of 109.50.
Now, in the given dewar structure of benzene, we can see that four carbon atoms are bonded with one double bond and two single bonds, and two carbon atoms are bonded with four single bonds with hydrogen and carbon.
Hence four carbons will exhibit sp2 hybridisation and two will exhibit sp3 hybridisation.
Therefore, option (C) is correct.
Note:
Dewar Benzene is the bicyclic isomer of benzene. It was given by James Dewar. In this structure, the carbon atoms form a tetrahedral geometry rather than making a planar structure. This is due to the fact that the carbon atom where the ring is joined is bonded to four atoms instead of three.
