Question
Question: In the given crystal structure what would be the cation X which replaces \(N{{a}^{+}}\) to create a ...
In the given crystal structure what would be the cation X which replaces Na+ to create a cation valency?
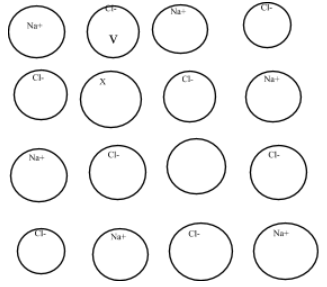
Solution
The crystal structure describes the Schottky defect in which a cation is missing from its crystal lattice and crystal as a whole is electrically neutral. So, X should be that cation with +2 charge so that the electrical neutrality of the crystal is maintained. Now answer the statement.
Complete answer:
The given crystal structure of sodium chloride represents the Schottky defect. Schottky defect is a point defect. This defect arises due to some of the atoms or ions which are missing from their normal lattice sites.
This type of defect is found in those stoichiometric compounds in which the number of the positive and negative ions are exactly in the same ratios as indicated by their chemical formulae.
Now considering the statement-
In the given structure of sodium chloride, we can see that there are eight chloride ions i.e., eight negative ions but on the contrary, there are 6 sodium ions i.e., six positive ions and two positive ions are missing from its place.
To maintain the electrical neutrality of the crystal structure and to create the cationic valency, the X should be that cation which carries +2.
In sodium chloride crystal structure, strontium ion is added to create the cationic valency as-
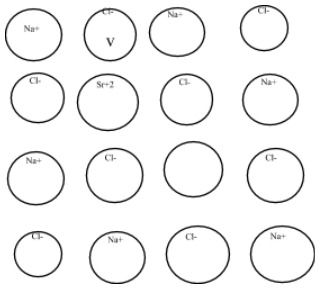
So, thus IN the given crystal structure what would be the cation of strontium Sr2+ which replaces Na+ to create a cation valency.
Note:
Schottky defect is shown by those ionic compounds which have high coordination number and in which the ions i.e., the anions and the cations have almost similar sizes.
