Question
Question: In the formation of alkene when alcoholic \(KOH\) reacts with \(C{H_3}C{H_2}Cl\) . What is the attac...
In the formation of alkene when alcoholic KOH reacts with CH3CH2Cl . What is the attacking species in the reaction?
A.C2H5O−
B.OH−
C.H3O+
D.K+
Solution
Dehydrohalogenation are those types of elimination reactions in which hydrogen and a halogen are eliminated. Attacking species include electrophile, nucleophile and free radicals.
Complete step by step answer:
When alkyl halides are heated in the presence of alcoholic potash (alcoholic potash is when potassium hydroxide is dissolved in alcohol), the product we get is an alkene.
In this one molecule of halogen is eliminated to give alkene.
This type of reaction is also known as dehydrohalogenation reaction.
This type of reaction is an example of β− elimination reaction where hydrogen from the β−carbon is eliminated and halogen from α− carbon is eliminated.
The general reaction of dehydrohalogenation is given as follows:
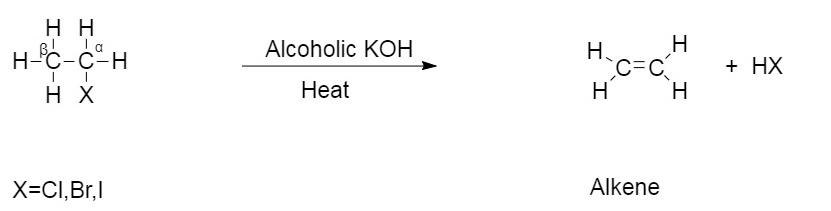
This is the reaction of chloroethane reacting in the presence of alcoholic KOH .
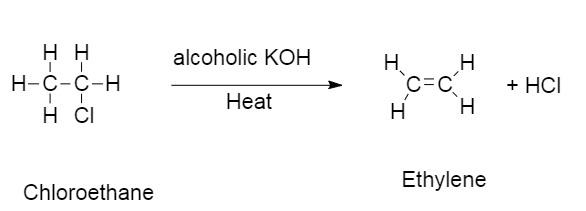
In this reaction chloroethane reacts with alcoholic KOH to give ethylene and HCl .
The hydrogen from the β− carbon and a halogen is displaced from chloroethane to give ethylene and hydrochloric acid.
The alcoholic KOH solution consists of alkoxide ion (RO−) which is a strong base. When alcohol KOH reacts with alkyl halide , the alkoxide easily abstracts hydrogen from the β−carbon atom and forms alkene by eliminating the molecule of hydrogen halide.
The attacking species that is present in this reaction is C2H5O− .
So the correct answer is option a) C2H5O− .
Note: If we use aqueous KOH in this reaction then the attacking species here will be OH− ion which is a weak base because it becomes weaker as it is highly solvated in aqueous solution. Thus it cannot abstract hydrogen from β− carbon atom easily. Therefore, OH− is a weaker base as compared to alkoxide ion.
