Question
Question: In the following the most stable conformation of n-butane is: A.
B.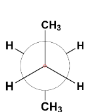
C.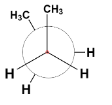
D.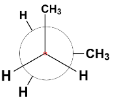
Solution
The conformer of n-butane in which the bulky groups are situated at the farthest possible position from each other has low steric hindrance and it is the most stable conformation.
Complete step by step answer: Conformational isomerism is a type of stereoisomerism that takes place due to the possible rotations around sigma bonds in a compound and the isomers produced due to such rotation is called conformational isomers or conformers. They are represented on a 2D plane with the help of Newman projection in which the front atom is denoted by a dot and the carbon at back is represented as a circle.
Now, the n-butane is an unsaturated hydrocarbon having a four-carbon chain. The 4 different conformational isomers of n-butane are possible due to the rotation around the sigma bond of C2 and C3 carbon atoms.
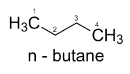
The four conformers of n-butane can be represented as follows:
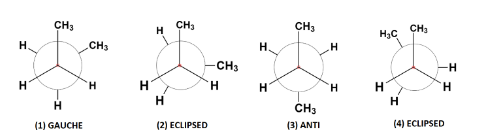
The first conformer is known as staggered conformation or more specifically gauche conformation of n-butane in which the two bulky methyl groups are situated at a dihedral angle (angle between two planes containing C – C bond) 60∘.
The second and the fourth conformers are called eclipsed conformation. It is the most unstable conformation because the two groups are present at a dihedral angle of 0∘ which increases the steric strain in the compound.
The third conformation is anti – conformation which is the most stable of all as the heavier methyl groups are situated opposite each other at a dihedral angle 180∘. This decreases the strain and stabilizes the compound.
Additional information: Each conformer is produced by rotations of 60∘ around the C – C sigma bond in butane and thus they are interconvertible to each other.
**Hence the correct answer is:
(B) 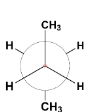
**
Note: The conformational isomers cannot be isolated at normal conditions due to their rapid conversions into each other. The increasing order of stability of conformations of n-butane is:
Eclipsed<Gauche<Anti
