Question
Question: In the following species, how many species have same magnetic moment? (i) Cr$^{2+}$ (ii) Mn$^{3+}$ ...
In the following species, how many species have same magnetic moment?
(i) Cr2+ (ii) Mn3+ (iii) Ni3+ (iv) Sc2+ (v) Zn2+ (vi) V3+ (vii) Ti+
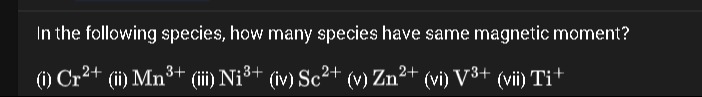
4
Solution
To find the number of species with the same magnetic moment, we first need to determine the number of unpaired electrons for each species. The spin-only magnetic moment is given by μs=n(n+2) BM, where n is the number of unpaired electrons. Species with the same number of unpaired electrons will have the same magnetic moment.
We write the electronic configuration for each neutral atom and then remove electrons according to the charge to find the configuration of the ion. Electrons are removed first from the outermost shell (highest principal quantum number) and then from the d-subshell.
(i) Cr (Z=24): [Ar]3d54s1. Cr2+: Remove 2 electrons (1 from 4s, 1 from 3d). Configuration: [Ar]3d4. Number of unpaired electrons n=4. (ii) Mn (Z=25): [Ar]3d54s2. Mn3+: Remove 3 electrons (2 from 4s, 1 from 3d). Configuration: [Ar]3d4. Number of unpaired electrons n=4. (iii) Ni (Z=28): [Ar]3d84s2. Ni3+: Remove 3 electrons (2 from 4s, 1 from 3d). Configuration: [Ar]3d7. Number of unpaired electrons n=3 (since d7 configuration has 2 paired and 3 unpaired electrons). (iv) Sc (Z=21): [Ar]3d14s2. Sc2+: Remove 2 electrons (2 from 4s). Configuration: [Ar]3d1. Number of unpaired electrons n=1. (v) Zn (Z=30): [Ar]3d104s2. Zn2+: Remove 2 electrons (2 from 4s). Configuration: [Ar]3d10. Number of unpaired electrons n=0. (vi) V (Z=23): [Ar]3d34s2. V3+: Remove 3 electrons (2 from 4s, 1 from 3d). Configuration: [Ar]3d2. Number of unpaired electrons n=2. (vii) Ti (Z=22): [Ar]3d24s2. Ti+: Remove 1 electron (1 from 4s). Configuration: [Ar]3d24s1. Number of unpaired electrons n=3 (2 in 3d and 1 in 4s).
Summary of the number of unpaired electrons (n) for each species:
- Cr2+: n=4
- Mn3+: n=4
- Ni3+: n=3
- Sc2+: n=1
- Zn2+: n=0
- V3+: n=2
- Ti+: n=3
Species with the same number of unpaired electrons will have the same magnetic moment.
- Cr2+ and Mn3+ both have n=4. Their magnetic moment is 4(4+2)=24 BM.
- Ni3+ and Ti+ both have n=3. Their magnetic moment is 3(3+2)=15 BM.
- Sc2+ has n=1. Magnetic moment 1(1+2)=3 BM.
- Zn2+ has n=0. Magnetic moment 0(0+2)=0 BM.
- V3+ has n=2. Magnetic moment 2(2+2)=8 BM.
The question asks "how many species have same magnetic moment?". This means we need to count the individual species from the list that have a magnetic moment identical to at least one other species in the list.
The species with n=4 are Cr2+ and Mn3+. Both have the same magnetic moment. These are 2 species. The species with n=3 are Ni3+ and Ti+. Both have the same magnetic moment. These are 2 species. The other species (Sc2+, Zn2+, V3+) have unique magnetic moments in this list.
The species that have the same magnetic moment as another species in the list are Cr2+, Mn3+, Ni3+, and Ti+. There are a total of 4 such species.
