Question
Question: In the following skew conformation of ethane, \({H^1} - C - C - {H^2}\) the dihedral angle is: ![]...
In the following skew conformation of ethane, H1−C−C−H2 the dihedral angle is:
A) 1200
B) 580
C) 1490
D) 1510
Explanation
Solution
The dihedral angle can be defined as an angle between the two planes of which are intersecting with each other. The atoms H1 and H2 show the dihedral angle with each other and by analyzing the plane of those atoms one can calculate the value of the dihedral angle.
Complete step by step answer:
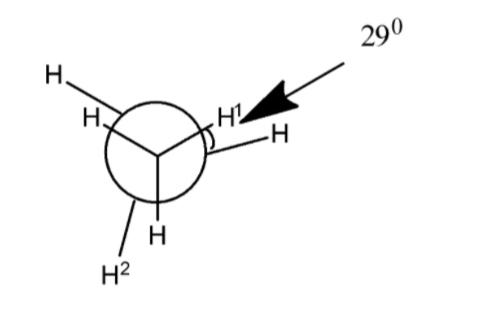
- In the above skew conformation of ethane, there are two carbon atoms connected with each other by a single sigma bond, the one in the center of three hydrogens and circle at the front which is visible and one behind the circle and at the center of the three hydrogen atoms which is not visible from the front but it is present in the back.
- A skew conformation is the type of arrangement of a molecule where the three atoms of one carbon atom are coplanar with the three atoms of the adjacent carbon atom.
- The dihedral angle which is to be calculated between H1−C−C−H2 and the two hydrogen atoms are located the two carbon atoms which are adjacent to each other and shows the inclination between them at an angle of more than 1200.
- When the three hydrogen atoms of one carbon are eclipsed to the tree hydrogen atoms of adjacent carbon atom each i.e. they are exactly in a plane with each other and the two eclipsed hydrogen atoms show 00 angle with each other and the noted hydrogen atoms H1 and H2 show the dihedral angle of 1200 with each other.
- But in the above skew conformation of ethane, it is shown that there is an angle of 290 in the two eclipsed hydrogen atoms which means we have to add the same value of the angle in the noted hydrogen dihedral angle.
- By considering the above factors,
Dihedral angle of H1−C−C−H2=1200+290=1490 - Therefore, the dihedral angle H1−C−C−H2 is 1490 which shows option C as the correct choice.
Note:
The dihedral angle is present between any of the three hydrogen atoms on one carbon and any one of the hydrogen atoms on another adjacent carbon atom. It is important to note that the dihedral angle doesn’t exist between the hydrogen atoms of the same carbon atom.
