Question
Question: In the following sequence of reactions: Toluene\(\xrightarrow{{KMn{O_4}}}\)A\(\xrightarrow{{SOC{l_2}...
In the following sequence of reactions: TolueneKMnO4ASOCl2BH2/PdBaSO4C; then product C is:
(A) C6H5COOH
(B) C6H5CH3
(C) C6H5CH2OH
(D) C6H5CHO
Solution
We should know that KMnO4 is an oxidising agent, SOCl2 is an acylating agent and H2/Pd or BaSO4 are reducing agents which cause the Rosenmund’s reduction.
Complete step by step answer:
-In this question we need to go step by step for each reactant and first find A, then B and then C as shown in the reaction: TolueneKMnO4ASOCl2BH2/PdBaSO4C
-Now let us begin by finding (A). We know that the initial reactant is toluene (C6H5−CH3) and KMnO4 is a strong oxidizing agent and it oxidizes toluene to benzoic acid. The reaction is shown below:
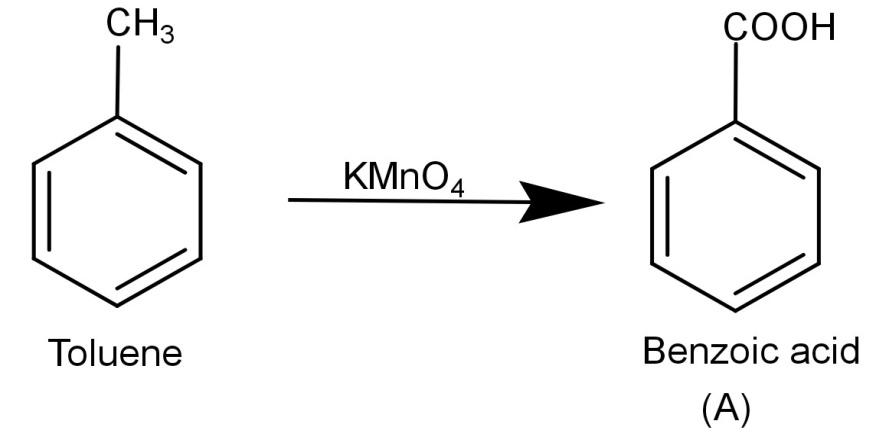
-Now we need to react (A) Benzoic acid with SOCl2 (Thionyl chloride). The thionyl chloride converts carboxylic acid to acid chloride and hence it will convert benzoic acid to benzoyl chloride. The reaction is shown below:
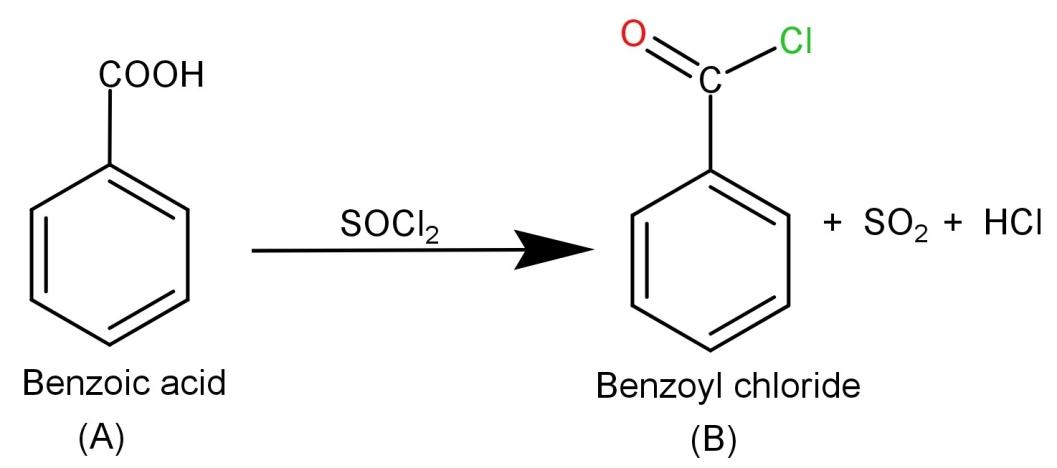
-Finally the product (B) Benzoyl chloride needs to be reacted with H2/Pd or BaSO4. They are reducing agents and hence Rosenmund reduction occurs which causes the conversion of benzoyl chloride to Benzaldehyde. So the final product is Benzaldehyde and the involved reaction is:
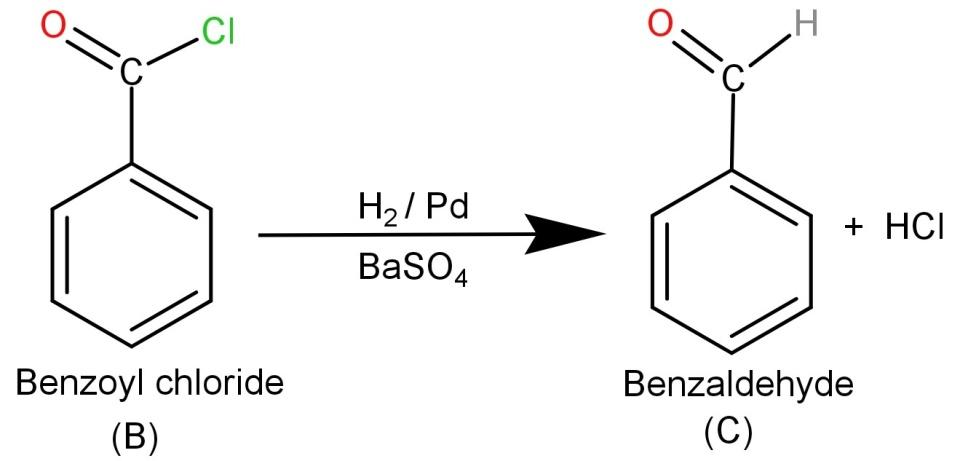
From this we know that (C) is Benzaldehyde (C6H5CHO)
So, the correct answer is “Option D”.
Note: Since Benzaldehyde is a colourless liquid with a characteristic almond like odour, it can be used as a bitter component of almond oil and is also used to impart artificial almond flavour to foods and some other scented products.
