Question
Question: In the following sequence of reaction, ...
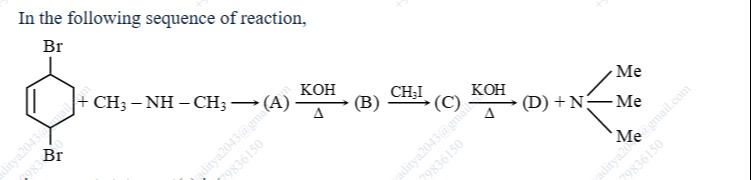
In the following sequence of reaction,
Answer
Benzene
Explanation
Solution
The sequence of reactions involves the reaction of 1,4-dibromocyclohex-2-ene with dimethylamine, followed by Hofmann elimination.
- 1,4-dibromocyclohex-2-ene reacts with dimethylamine (CH3-NH-CH3) via nucleophilic substitution of one bromine atom to form (1-(dimethylamino)cyclohex-2-en-4-yl)bromide (A).
- Heating (A) with KOH causes intramolecular elimination of HBr to form 1-(dimethylamino)cyclohexa-2,4-diene (B).
- Reaction of the tertiary amine (B) with methyl iodide (CH3I) leads to the formation of the quaternary ammonium iodide (1-(trimethylammonium)cyclohexa-2,4-diene) iodide (C).
- Heating the quaternary ammonium hydroxide (formed from (C) and KOH) causes Hofmann elimination, leading to the formation of benzene (D) and trimethylamine (N(CH3)3). The elimination occurs by removing a proton from C6, forming a double bond between C1 and C6, which results in the formation of the stable aromatic ring of benzene.
