Question
Question: In the following reactions, products A and B are: 
A.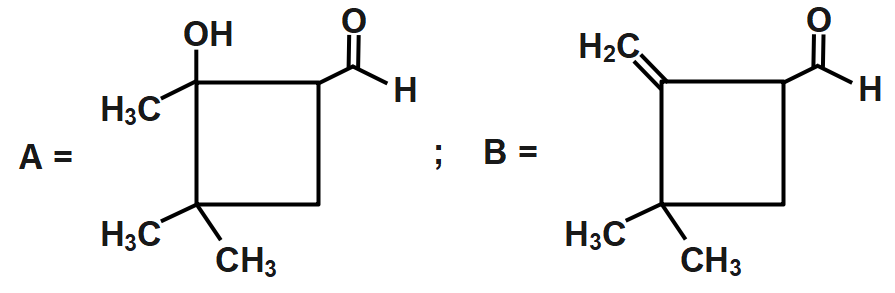
B.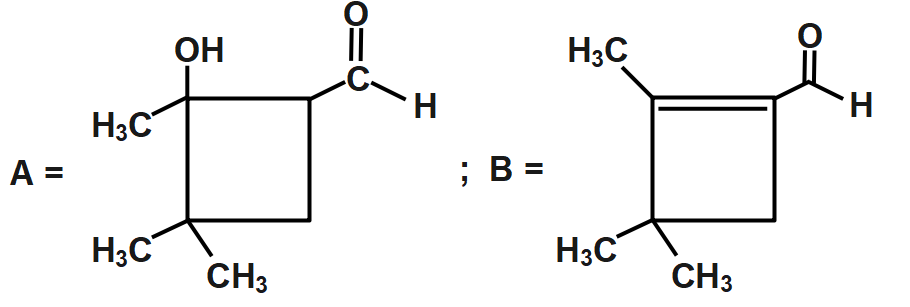
C.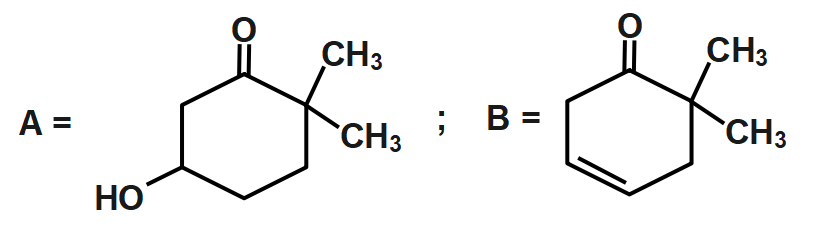
D.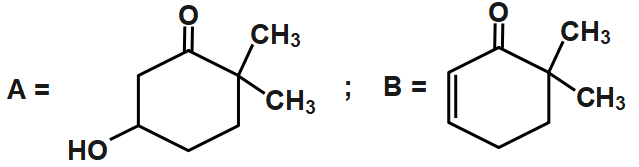
Solution
The answer here is dependent on the basic concept of organic chemistry which deals with the reaction mechanism where aldol condensation gives the β hydroxy aldehyde or β hydroxy ketone and decarboxylation is the removal of carboxyl group. Aldol condensation is an organic reaction.
Complete step by step answer:
In an aldol condensation reaction, the aldehydes and ketones having at least one α− hydrogen participate in the presence of a catalyst. The catalyst in the Aldol condensation reaction is the dilute alkali. The example of aldol condensation in which the ethanal that is one of the aldehyde in the presence of dilute alkali acid that is gives an aldol product.
Aldol condensation as the name itself says is the condensation of an enol or enolate ion with the carbonyl compound to form a hydroxy aldehyde or hydroxy ketone which is followed by dehydration to give a conjugated enone. The chemical reaction is shown below:
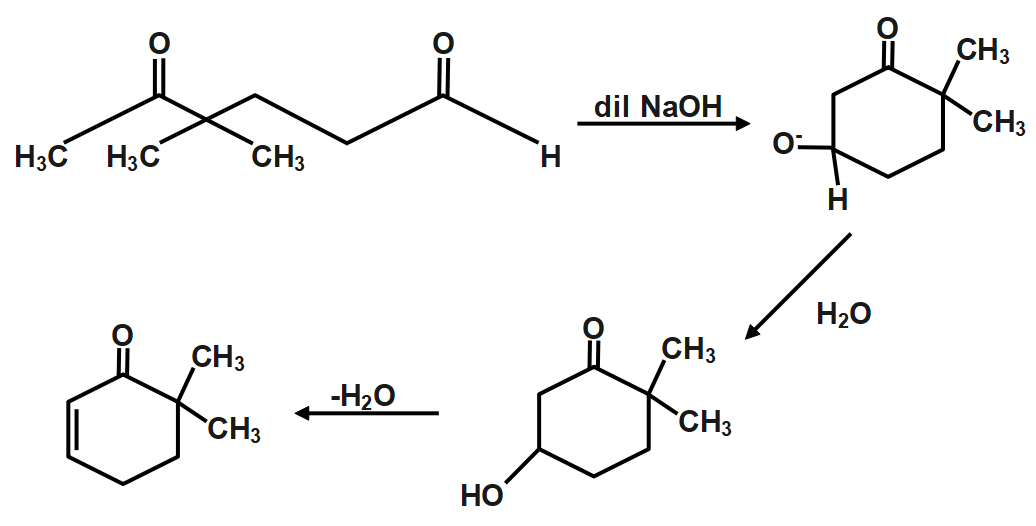
In an aldol condensation reaction, both the aldol and ketol formed readily lose water and the aldol condensation product, i.e. α, β− unsaturated carbonyl compounds are formed. This reaction's name has been derived from the functional groups, namely aldehyde and alcohol present in the reaction products.
Hence, the Aldol condensation reaction is an organic reaction in which a carbonyl compound reacts with enolate ion and leads to the formation of the β− hydroxy aldehyde or β− hydroxy ketone. Thus, aldol condensation reaction occurs when aldehydes or ketones have at least one α− hydrogen reaction in the presence of a dilute alkali.
Note: But when aldol condensation is carried out between two different ketones or aldehydes, this reaction is termed as the cross aldol condensation reaction. The aldol condensation takes place only if there is a α− hydrogen atom because the aldol condensation requires the formation of the enolate ion or enol and the absence of α− hydrogen does not give these intermediates.
