Question
Question: In the following reaction \[{\text{Mn}}{{\text{O}}_{\text{2}}}{\text{ + HCl }} \to {\text{ MnC}}{{\t...
In the following reaction MnO2 + HCl → MnCl2 + H2O + Cl2
Find
-Substance reduced
-Substance oxidized
-Oxidizing agent
-Reducing agent
Solution
Assign the oxidation number to all atoms. Oxidation is the loss of electrons while reduction is the gain of electrons. The substance which helps other species to get reduced and itself get oxidized is known as a reducing agent. While substance that helps other species to get oxidised and itself get reduced is known as oxidising agent
Complete Step by step answer: he redox reaction given to us is:
MnO2 + HCl → MnCl2 + H2O + Cl2
Using the oxidation number rules we can calculate the oxidation number of all atoms in the given reaction as follows:
The oxidation number of oxygen is always -2 except in peroxide. In peroxide oxidation number of oxygen is -1.
Using this rule we can determine the oxidation number of Mn in [{\text{Mn}}{{\text{O}}_{\text{2}}}{\text{ }}].
Oxidation number of O =-2
So, Oxidation number of Mn in MnO2 = +4
Oxidation number of hydrogen is always +1 except in metal hydride it is -1.
Oxidation number of chlorine is -1 in most of the compounds.
Oxidation number of H in HCl = +1
So, oxidation number of Cl in HCl = -1
As oxidation number of Clis -1 so oxidation number of Mn in MnCl2 is +2.
Oxidation number of H in H2O is +1 and oxidation number of O is -2.
Oxidation number of an element is zero so oxidation number of Cl in Cl2 is 0.
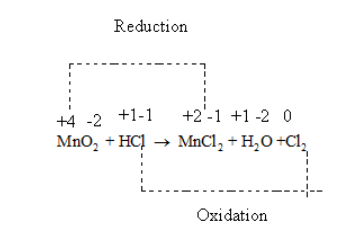
Thus, in the given reaction Mn is getting reduced from +4 to +2 and Cl is getting oxidised from -1 to 0.
So,
The substance reduce isMnO2
The substance oxidised isHCl .
The substance that is reduced acts as an oxidising agent while a substance that is oxidised acts as a reducing agent.
Hence, Oxidising agent = MnO2
Reducing agent = HCl
Note: In the given reactionMnO2 is getting reduced and oxidised HCl so act as oxidising agent. While HCl is getting oxidised and reduced MnO2so act as reducing agent. To determine the species getting oxidised and reduced it is important to assign the oxidation number correctly.
