Question
Question: In the following reaction, \({{\text{C}}_{6}}{{\text{H}}_{5}}\text{CHO + C}{{\text{H}}_{3}}\text{COC...
In the following reaction, C6H5CHO + CH3COCH3OH−Δ P which product is formed?
A. C6H5COH
B. C6H5-CO-C6H5
C. C6H5CH=CHCOCH3
D. C6H5−CO - CO - C6H5
Solution
The reaction is an example of an aldol condensation. Aldol condensation is a reaction in which the carbonyl group reacts with the enol group yielding beta-hydroxy aldehyde/ ketone. This is a type of condensation reaction.
Complete answer:
-In the given reaction, the reactants are aldehyde and ketone and we have to find the product P formed when they both will react.
-They both will undergo aldol condensation reaction in which the following steps are followed:
Step I: firstly, the hydroxyl ion will deprotonate the aldehyde group by breaking the bond between carbon and hydrogen and releases enol ion and water i.e.
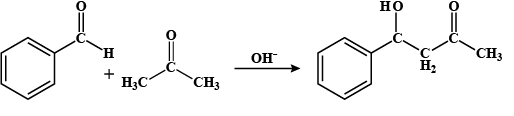
Benzaldehyde Acetone 4 - hydroxy -4-phenyl but-2-one
Step II: Now, after deprotonation, the ketone attaches to the aldehyde group and forms 4 - hydroxy -4-phenyl but-2-one
Step III: Now, it will be heated and undergoes a dehydration process in which the water is released, by removal of a hydroxyl group from benzaldehyde and hydrogen from ketone that is attached to the benzaldehyde.
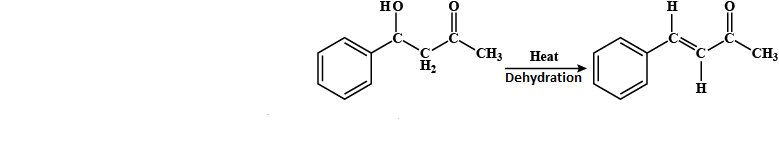
4 - hydroxy -4-phenyl but-2-one 4-phenyl but-3-ene-2-one
-So, the product P formed is C6H5CH=CHCOCH3.
So, the correct answer is “Option C”.
Note: For an aldol condensation to take place the compound should have an alpha-hydrogen i.e. hydrogen which is attached directly to the functional group. Crossed aldol condensation is the reaction in which the two different aldehyde or ketone compounds react with each other.
.
